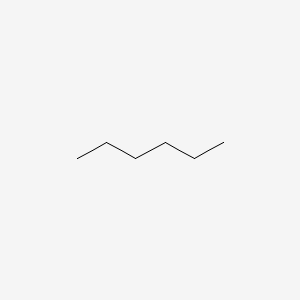
Tin iv hydroxide
Tin Iv Hydroxide. The compound is often encountered as one of its hydrates FeOOH n H 2 O rust. The monohydrate FeOOH H 2 O CAS CI. C 4 H 10 O. 77492 is often referred to as ironIII hydroxide FeOH 3 hydrated iron oxide yellow iron oxide or Pigment Yellow 42.
 Ionic Compounds Using Polyatomic Ions Ppt Download From slideplayer.com
Ionic Compounds Using Polyatomic Ions Ppt Download From slideplayer.com
Polyatomic Ions Worksheet Answer Key. Ionic bonds also melt at high temperatures. The structure of the bond is rigid strong and often crystalline and solid. For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atomsThe atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit. 77492 is often referred to as ironIII hydroxide FeOH 3 hydrated iron oxide yellow iron oxide or Pigment Yellow 42. NH 4 ammonium.
Polyatomic ions are ions which consist of more than one atom.
77492 is often referred to as ironIII hydroxide FeOH 3 hydrated iron oxide yellow iron oxide or Pigment Yellow 42. H 2 PO 4-Sulfate. HPO 4 2-Dihydrogen phosphate. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. The structure of the bond is rigid strong and often crystalline and solid. Negative ions that consist of a single atom are named by adding the suffix -ide to the stem of the name of the element.

Complete these in lab and on your own time for practice. Name or write the. For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atomsThe atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit. HPO 4 2-Dihydrogen phosphate. 77492 is often referred to as ironIII hydroxide FeOH 3 hydrated iron oxide yellow iron oxide or Pigment Yellow 42.
 Source: slideplayer.com
Source: slideplayer.com
AsO 3 3-Hydrogen phosphate. H 2 PO 4-Sulfate. Tin IV tin II Sb3 Sb5 antimonyIII antimonyV Tc7 technitium Ta5 tantalum W6 tungsten Re7 rhenium Os4 osmium Ir4 iridium 87 88 89 H hydrogen Li lithium Be2 beryllium Na sodium Mg2 magnesium K potassium Ca2 Rb rubidium Sr2 strontium Cs cesium Ba2 calcium barium Fr francium Ra2 radium B boron C carbon nitride N3-oxide O2-fluoride F-neon Ne Al3 aluminum Si. Ions grouped by charge anions grouped by periodic position Simple ions. Complete these in lab and on your own time for practice.

Cl - chloride OH - hydroxide F -. Ionic bonds also melt at high temperatures. Co 3 3 sodium Na 1 hydroxide OH 1 cobalt IV Co 4 4 tin II stannous Sn 2 2 iodate IO 3 1 copper I cuprous Cu 1 tin IV stannic Sn 4 4 iodide I 1 copper II cupric Cu 2 2 titanium II Ti 2 2 nitrate NO 3 1 gold I aurous Au 1 titanium III Ti 3 3 nitrite NO 2 1 gold III auric Au 3 3 titanium IV Ti 4 4 nitride. Acetic acid acetic aldehyde acetone. For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atomsThe atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit.
 Source: youtube.com
Source: youtube.com
AsO 3 3-Hydrogen phosphate. Ionic bonds also melt at high temperatures. Co 3 3 sodium Na 1 hydroxide OH 1 cobalt IV Co 4 4 tin II stannous Sn 2 2 iodate IO 3 1 copper I cuprous Cu 1 tin IV stannic Sn 4 4 iodide I 1 copper II cupric Cu 2 2 titanium II Ti 2 2 nitrate NO 3 1 gold I aurous Au 1 titanium III Ti 3 3 nitrite NO 2 1 gold III auric Au 3 3 titanium IV Ti 4 4 nitride. Check our FAQ section for more details. A related more well-characterized NiIII-based material is nickel oxide hydroxide NiOOH which is likely the reagent employed in organic synthesis since it is generated in aqueous.
 Source: researchgate.net
Source: researchgate.net
Negative ions that consist of a single atom are named by adding the suffix -ide to the stem of the name of the element. Name or write the. Tin IV tin II Sb3 Sb5 antimonyIII antimonyV Tc7 technitium Ta5 tantalum W6 tungsten Re7 rhenium Os4 osmium Ir4 iridium 87 88 89 H hydrogen Li lithium Be2 beryllium Na sodium Mg2 magnesium K potassium Ca2 Rb rubidium Sr2 strontium Cs cesium Ba2 calcium barium Fr francium Ra2 radium B boron C carbon nitride N3-oxide O2-fluoride F-neon Ne Al3 aluminum Si. H 3 O hydronium. For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atomsThe atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit.

SO 4 2. A related more well-characterized NiIII-based material is nickel oxide hydroxide NiOOH which is likely the reagent employed in organic synthesis since it is generated in aqueous. AsO 3 3-Hydrogen phosphate. The monohydrate FeOOH H 2 O CAS CI. 5 tin IV selenide 6 gallium arsenide 7 lead IV sulfate 8 beryllium bicarbonate 9 manganese III sulfite 10 aluminum cyanide 11 CrPO 4 2 12 VCO 3 2 13 SnNO 2 2 14 Co 2 O 3 15 TiC 2 H 3 O 2 2 16 V 2 S 5 17 CrOH 3 18 LiI 19 Pb 3 N 2 20 AgBr 21 NaBr sodium bromide 22 ScOH 3 scandium III hydroxide 23 V 2 SO 4.
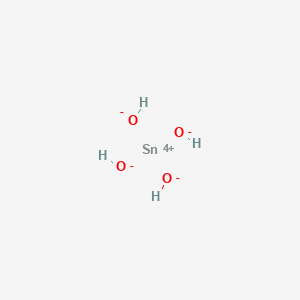
H 2 PO 4-Sulfate. Different values of the pure water 0 concentration density reflect the fact that the measurements were done in different temperatures. Cl - chloride OH - hydroxide F -. Acetic acid acetic aldehyde acetone. IronIII oxide-hydroxide or ferric oxyhydroxide is the chemical compound of iron oxygen and hydrogen with formula FeOOH.
 Source: slideplayer.com
Source: slideplayer.com
This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. For example nitrate ion NO 3- contains one nitrogen atom and three oxygen atomsThe atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit. Co 3 3 sodium Na 1 hydroxide OH 1 cobalt IV Co 4 4 tin II stannous Sn 2 2 iodate IO 3 1 copper I cuprous Cu 1 tin IV stannic Sn 4 4 iodide I 1 copper II cupric Cu 2 2 titanium II Ti 2 2 nitrate NO 3 1 gold I aurous Au 1 titanium III Ti 3 3 nitrite NO 2 1 gold III auric Au 3 3 titanium IV Ti 4 4 nitride. However this group of atoms is most stable when it has either lost of gained an electron and thus existed as a charged ion. HPO 4 2-Dihydrogen phosphate.

There are a number of ions that are not individual atoms but are composed of multiple atoms that are covalently bonded together. H 3 O hydronium. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Name or write the. There are a number of ions that are not individual atoms but are composed of multiple atoms that are covalently bonded together.
 Source: youtube.com
Source: youtube.com
Negative ions that consist of a single atom are named by adding the suffix -ide to the stem of the name of the element. H 2 PO 4-Sulfate. The corrosion data in this section is mainly based on the results of general corrosion laboratory tests which are not strictly comparable with actual service conditionsThe corrosion tables provide an initial guide to the selection of materials and are intended to facilitate understanding of the different types of corrosion damage that can arise due to poor material selection. Tin IV tin II Sb3 Sb5 antimonyIII antimonyV Tc7 technitium Ta5 tantalum W6 tungsten Re7 rhenium Os4 osmium Ir4 iridium 87 88 89 H hydrogen Li lithium Be2 beryllium Na sodium Mg2 magnesium K potassium Ca2 Rb rubidium Sr2 strontium Cs cesium Ba2 calcium barium Fr francium Ra2 radium B boron C carbon nitride N3-oxide O2-fluoride F-neon Ne Al3 aluminum Si. Cl - chloride OH - hydroxide F -.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title tin iv hydroxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.