Tin iv oxide
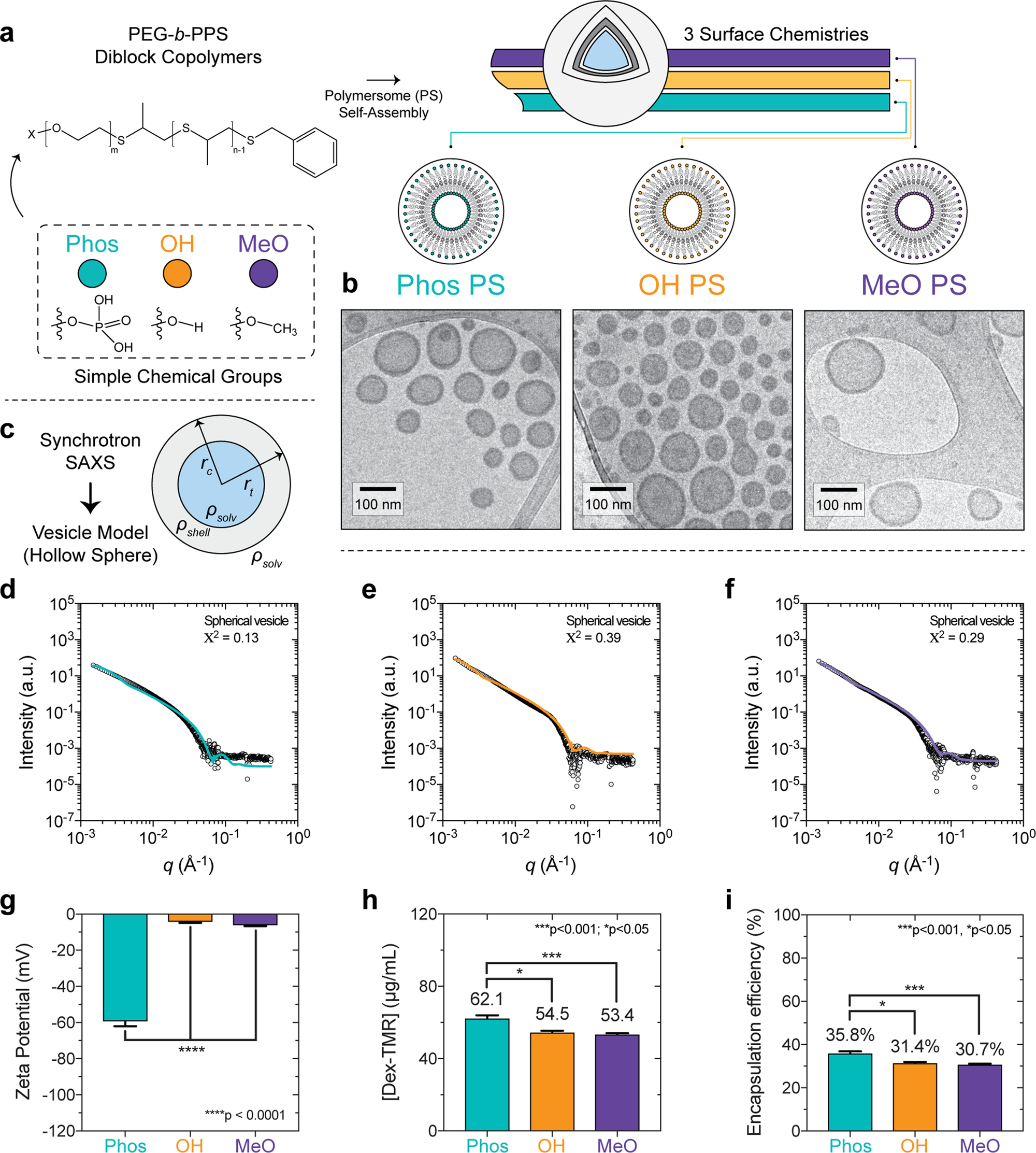
Tin Iv Oxide. It has tin in its 4 oxidation state. Its chemical formula is SnO 2. The mineral form of SnO 2 is called cassiterite and this is the main ore of tin. E - læring er den enkleste måten å holde seg faglig oppdatert på fleksibelt rimelig og effektivtKursene fra Norsk Helseinformatikk er godkjent av Legeforeningen og Sykepleierforeningen.
 How To Write The Formula For Tin Iv Oxide Youtube From youtube.com
How To Write The Formula For Tin Iv Oxide Youtube From youtube.com
TinII oxide burns in air with a dim green flame to form SnO 2. It is a white powdery solid. E - læring er den enkleste måten å holde seg faglig oppdatert på fleksibelt rimelig og effektivtKursene fra Norsk Helseinformatikk er godkjent av Legeforeningen og Sykepleierforeningen. Find chemical and physical properties biological activities safety and toxicity information patents literature citations and more. Tin IV oxide fibers optical microscope TinIV oxide crystallises with. TinIV oxide also known as stannic oxide is the inorganic compound with the formula SnO 2.
It reacts with acids to produce stannic salts.
It is a colourless diamagnetic amphoteric solid. It has tin in its 4 oxidation state. Its chemical formula is SnO 2. Search chemicals by name molecular formula structure and other identifiers. When heated in an inert atmosphere initially disproportionation occurs giving Sn metal and Sn 3 O 4 which further reacts to give SnO 2 and Sn metal. Tin IV oxide fibers optical microscope TinIV oxide crystallises with.
 Source: fishersci.se
Source: fishersci.se
TinIV oxide also known as tin dioxide or stannic oxide is a chemical compound. We would like to show you a description here but the site wont allow us. 2 SnO O 2 2 SnO 2. It is a white powdery solid. TinIV oxide also known as stannic oxide is the inorganic compound with the formula SnO 2.
 Source: en.wikipedia.org
Source: en.wikipedia.org
PubChem is the worlds largest collection of freely accessible chemical information. Tin IV oxide fibers optical microscope TinIV oxide crystallises with. It reacts with acids to produce stannic salts. SnO is amphoteric dissolving in strong acid to give tinII salts and in strong base to give stannites. It has tin and oxide ions in it.
 Source: gelest.com
Source: gelest.com
It has tin in its 4 oxidation state. The mineral form of SnO 2 is called cassiterite and this is the main ore of tin. With many other names this oxide of tin is an important material in tin chemistry. We would like to show you a description here but the site wont allow us. It has tin in its 4 oxidation state.
 Source: researchgate.net
Source: researchgate.net
SnO is amphoteric dissolving in strong acid to give tinII salts and in strong base to give stannites. 4SnO Sn 3 O 4 Sn Sn 3 O 4 2SnO 2 Sn. With many other names this oxide of tin is an important material in tin chemistry. It is a colourless diamagnetic amphoteric solid. 2 SnO O 2 2 SnO 2.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
2 SnO O 2 2 SnO 2. It has tin and oxide ions in it. TinII oxide burns in air with a dim green flame to form SnO 2. When heated in an inert atmosphere initially disproportionation occurs giving Sn metal and Sn 3 O 4 which further reacts to give SnO 2 and Sn metal. Search chemicals by name molecular formula structure and other identifiers.
 Source: coleparmer.com
Source: coleparmer.com
Find chemical and physical properties biological activities safety and toxicity information patents literature citations and more. Its chemical formula is SnO 2. We would like to show you a description here but the site wont allow us. We would like to show you a description here but the site wont allow us. It is a colourless diamagnetic amphoteric solid.
 Source: youtube.com
Source: youtube.com
2 SnO O 2 2 SnO 2. We would like to show you a description here but the site wont allow us. It is a colourless diamagnetic amphoteric solid. Find chemical and physical properties biological activities safety and toxicity information patents literature citations and more. When heated in an inert atmosphere initially disproportionation occurs giving Sn metal and Sn 3 O 4 which further reacts to give SnO 2 and Sn metal.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
When heated in an inert atmosphere initially disproportionation occurs giving Sn metal and Sn 3 O 4 which further reacts to give SnO 2 and Sn metal. We would like to show you a description here but the site wont allow us. It reacts with acids to produce stannic salts. It is a white powdery solid. E - læring er den enkleste måten å holde seg faglig oppdatert på fleksibelt rimelig og effektivtKursene fra Norsk Helseinformatikk er godkjent av Legeforeningen og Sykepleierforeningen.
 Source: ereztech.com
Source: ereztech.com
It is a white powdery solid. It has tin in its 4 oxidation state. It has tin and oxide ions in it. The mineral form of SnO 2 is called cassiterite and this is the main ore of tin. 4SnO Sn 3 O 4 Sn Sn 3 O 4 2SnO 2 Sn.
 Source: youtube.com
Source: youtube.com
4SnO Sn 3 O 4 Sn Sn 3 O 4 2SnO 2 Sn. The mineral form of SnO 2 is called cassiterite and this is the main ore of tin. It is a colourless diamagnetic amphoteric solid. It is a white powdery solid. 4SnO Sn 3 O 4 Sn Sn 3 O 4 2SnO 2 Sn.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title tin iv oxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.