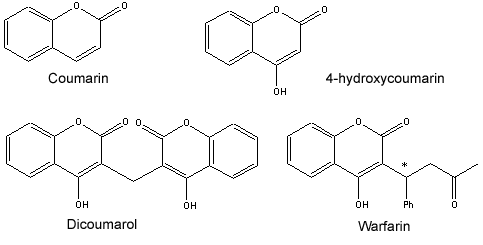
Titanium chloride molar mass
Titanium Chloride Molar Mass. Titanium tetrachloride is the inorganic compound with the formula TiCl 4It is an important intermediate in the production of titanium metal and the pigment titanium dioxideTiCl 4 is a volatile liquid. One formula unit of sodium chloride. UN 1789 solution Hydrochloric acid ACS reagent 37. Acido clorhidrico Spanish Varley Poly-Pak Bowl.
 Answered A 2 836g Titanium Chloride Compound Was Bartleby From bartleby.com
Answered A 2 836g Titanium Chloride Compound Was Bartleby From bartleby.com
Vanadium Reference Standard Solution. UN 1789 solution Hydrochloric acid ACS reagent 37. Titanium Reference Standard Solution. Acido clorhidrico Spanish Varley Poly-Pak Bowl. For example the atomic mass of titanium is 4788 amu or 4788 gmol. Thus since the atomic mass of iron is 55847 amu one mole of iron atoms would weigh 55847 grams.
Acido cloridrico Italian Platinum Cobalt Color Standard Solution.
The same concept can be extended to ionic compounds and molecules. UN 1050 anhydrous mono hydrochloride. Vanadium Reference Standard Solution. One formula unit of sodium chloride. The atomic mass is useful in chemistry when it is paired with the mole concept. The same concept can be extended to ionic compounds and molecules.
 Source: britannica.com
Source: britannica.com
Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol. Vanadium Reference Standard Solution. One formula unit of sodium chloride. Acido clorhidrico Spanish Varley Poly-Pak Bowl. UN 1050 anhydrous mono hydrochloride.
 Source: bartleby.com
Source: bartleby.com
One formula unit of sodium chloride. In 4788 grams of titanium there is one mole or 6022 x 10 23 titanium atoms. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Upon contact with humid air it forms spectacular opaque clouds of titanium dioxide TiO 2 and hydrated hydrogen chlorideIt is sometimes referred to as tickle or tickle 4 due to the. Thus since the atomic mass of iron is 55847 amu one mole of iron atoms would weigh 55847 grams.
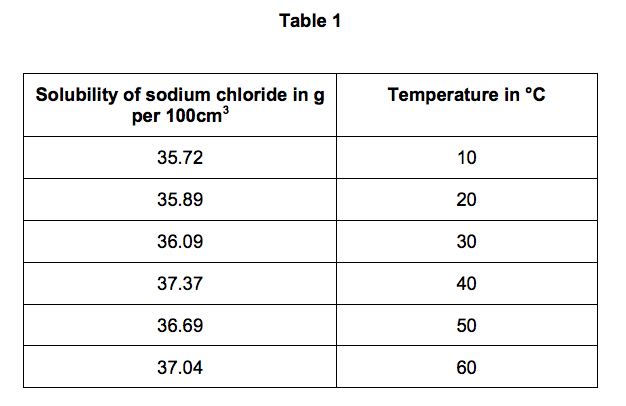 Source: elevise.co.uk
Source: elevise.co.uk
Hydrogen Chloride - Methanol Reagent. Titanium tetrachloride is the inorganic compound with the formula TiCl 4It is an important intermediate in the production of titanium metal and the pigment titanium dioxideTiCl 4 is a volatile liquid. For example the atomic mass of titanium is 4788 amu or 4788 gmol. Vanadium Reference Standard Solution. The atomic mass is useful in chemistry when it is paired with the mole concept.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Titanium Reference Standard Solution. White Emulsion Bowl Cleaner. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. One formula unit of sodium chloride. Thus since the atomic mass of iron is 55847 amu one mole of iron atoms would weigh 55847 grams.
 Source: youtube.com
Source: youtube.com
Vanadium Reference Standard Solution. UN 1789 solution Hydrochloric acid ACS reagent 37. In 4788 grams of titanium there is one mole or 6022 x 10 23 titanium atoms. For example the atomic mass of titanium is 4788 amu or 4788 gmol. Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol.
 Source: bartleby.com
Source: bartleby.com
The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element. Molar Mass of Frequently Calculated Chemicals. Acido clorhidrico Spanish Varley Poly-Pak Bowl. White Emulsion Bowl Cleaner. Thus since the atomic mass of iron is 55847 amu one mole of iron atoms would weigh 55847 grams.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
TinII chloride also known as stannous chloride is a white crystalline solid with the formula The template Tin is being considered for deletion Sn The template Chlorine is being considered for deletion Cl 2It forms a stable dihydrate but aqueous solutions tend to undergo hydrolysis particularly if hotSnCl 2 is widely used as a reducing agent in acid solution and in. Hydrogen Chloride - Methanol Reagent. The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element. Molar Mass of Frequently Calculated Chemicals. Molar mass is the mass of a given substance divided by the amount of that substance measured in gmol.
 Source: slideplayer.com
Source: slideplayer.com
Acido clorhidrico Spanish Varley Poly-Pak Bowl. The same concept can be extended to ionic compounds and molecules. Thus since the atomic mass of iron is 55847 amu one mole of iron atoms would weigh 55847 grams. Acido cloridrico Italian Platinum Cobalt Color Standard Solution. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver.

The same concept can be extended to ionic compounds and molecules. Hydrogen Chloride - Methanol Reagent. Titanium tetrachloride is the inorganic compound with the formula TiCl 4It is an important intermediate in the production of titanium metal and the pigment titanium dioxideTiCl 4 is a volatile liquid. One formula unit of sodium chloride. Upon contact with humid air it forms spectacular opaque clouds of titanium dioxide TiO 2 and hydrated hydrogen chlorideIt is sometimes referred to as tickle or tickle 4 due to the.

Acido cloridrico Italian Platinum Cobalt Color Standard Solution. UN 1789 solution Hydrochloric acid ACS reagent 37. One formula unit of sodium chloride. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. TinII chloride also known as stannous chloride is a white crystalline solid with the formula The template Tin is being considered for deletion Sn The template Chlorine is being considered for deletion Cl 2It forms a stable dihydrate but aqueous solutions tend to undergo hydrolysis particularly if hotSnCl 2 is widely used as a reducing agent in acid solution and in.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title titanium chloride molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.