Trisodium citrate dihydrate solubility
Trisodium Citrate Dihydrate Solubility. Citrate is used notably for elution in affinity chromatography but also for cell media. 18 The current specific absorption value for alpha-tocopherol E 307 should be corrected and the sublimation point for sorbic acid E 200 should be replaced by a solubility test as the former is not relevant. 01M citric acid monohydrate C 6 H 8 O 7 H 2 O FW 21014 Solution B. Citrate may also exert an anticoagulant effect via a so far unknown mechanism as restoration of calcium concentration does not fully reverse the effect of citrate 1.
 Sodium Citrate Dihydrate Cas 6132 04 3 Chemsrc From chemsrc.com
Sodium Citrate Dihydrate Cas 6132 04 3 Chemsrc From chemsrc.com
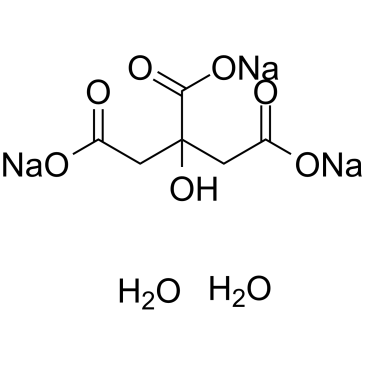
Trisodium citrate dihydrate is a tribasic salt of citric acid. 18 The current specific absorption value for alpha-tocopherol E 307 should be corrected and the sublimation point for sorbic acid E 200 should be replaced by a solubility test as the former is not relevant. It is widely used in foods beverages and various technical applications mainly. Trisodium phosphate TSP Na3PO4 is a useful cleaning agent but it must be handled with care because its solutions are quite caustic. Anhydrous salts are obtained by dehydration in rotary dryers or directly from the solutions by spray drying or in rotary kilns. Write molecular ionic and net ionic equations for this reaction.
01M citric acid monohydrate C 6 H 8 O 7 H 2 O FW 21014 Solution B.
Citrate is used notably for elution in affinity chromatography but also for cell media. The phosphates crystallize from the solutions as hydrates and are separated by centrifugation. Citrate is a weak base and so reacts with hydrochloric acid in the stomach to raise the pH. Drying conditions for trisodium citrate E 331 iii should also be adjusted to improve the reproducibility of the method. 18 The current specific absorption value for alpha-tocopherol E 307 should be corrected and the sublimation point for sorbic acid E 200 should be replaced by a solubility test as the former is not relevant. Preparation of Citrate Buffer pH 3062 To create 100ml of a 01M citrate buffer mix citric acid monohydrate and trisodium citrate dehydrate as given below.

Trisodium phosphate TSP Na3PO4 is a useful cleaning agent but it must be handled with care because its solutions are quite caustic. It commonly refers to the dihydrate type when used as a flavoring agent buffer chelating agent emulsifier stabilizer and preservative in food. Trisodium citrate dihydrate is a tribasic salt of citric acid. Citric Acid UP168781 1 Kg MW1921 Citric Acid ACS grade Biotech grade 673410 500 g MW1921 Citric Acid Trisodium Dihydrate 218830 1 Kg 218831 25 Kg MW2941 Citric Acid Trisodium Dihydrate Proteomics Grade 10853A 500 g 10853B 1 Kg. It has two forms trisodium citrate dihydrate and anhydrous.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
The specification of bacterial sources for the. Citrate is a weak base and so reacts with hydrochloric acid in the stomach to raise the pH. Citric Acid UP168781 1 Kg MW1921 Citric Acid ACS grade Biotech grade 673410 500 g MW1921 Citric Acid Trisodium Dihydrate 218830 1 Kg 218831 25 Kg MW2941 Citric Acid Trisodium Dihydrate Proteomics Grade 10853A 500 g 10853B 1 Kg. Anhydrous salts are obtained by dehydration in rotary dryers or directly from the solutions by spray drying or in rotary kilns. It commonly refers to the dihydrate type when used as a flavoring agent buffer chelating agent emulsifier stabilizer and preservative in food.
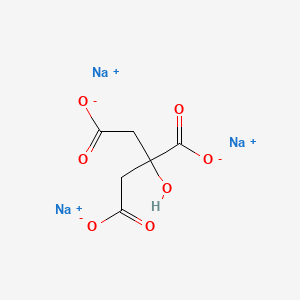
If a solution of Na3PO4 is added to one containing a calcium salt such as CaCl2 a precipitate of calcium phosphate is formed. The specification of bacterial sources for the. Milk of magnesia is a suspension of solid magnesium hydroxide. Sodium citrate is the sodium salt of citric acidIt is white crystalline powder or white granular crystals slightly deliquescent in moist air freely soluble in water practically insoluble in alcoholLike citric acid it has a sour tasteFrom the medical point of view it is used as alkalinizing agent. It it further metabolized to bicarbonate which then acts as a systemic alkalizing agent raising the pH of the blood and urine.
 Source: sciencedirect.com
Source: sciencedirect.com
The specification of bacterial sources for the. Citric Acid UP168781 1 Kg MW1921 Citric Acid ACS grade Biotech grade 673410 500 g MW1921 Citric Acid Trisodium Dihydrate 218830 1 Kg 218831 25 Kg MW2941 Citric Acid Trisodium Dihydrate Proteomics Grade 10853A 500 g 10853B 1 Kg. Trisodium phosphate TSP Na3PO4 is a useful cleaning agent but it must be handled with care because its solutions are quite caustic. Sodium citrate is the sodium salt of citric acidIt is white crystalline powder or white granular crystals slightly deliquescent in moist air freely soluble in water practically insoluble in alcoholLike citric acid it has a sour tasteFrom the medical point of view it is used as alkalinizing agent. If a solution of Na3PO4 is added to one containing a calcium salt such as CaCl2 a precipitate of calcium phosphate is formed.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The basicity of sodium carbonate is insufficient for the formation of trisodium phosphate so that caustic soda must be used in this step. Citric Acid UP168781 1 Kg MW1921 Citric Acid ACS grade Biotech grade 673410 500 g MW1921 Citric Acid Trisodium Dihydrate 218830 1 Kg 218831 25 Kg MW2941 Citric Acid Trisodium Dihydrate Proteomics Grade 10853A 500 g 10853B 1 Kg. Milk of magnesia is a suspension of solid magnesium hydroxide. The phosphates crystallize from the solutions as hydrates and are separated by centrifugation. Trisodium citrate dihydrate is a tribasic salt of citric acid.
 Source: chemsrc.com
Source: chemsrc.com
If a solution of Na3PO4 is added to one containing a calcium salt such as CaCl2 a precipitate of calcium phosphate is formed. Citrate is a weak base and so reacts with hydrochloric acid in the stomach to raise the pH. 18 The current specific absorption value for alpha-tocopherol E 307 should be corrected and the sublimation point for sorbic acid E 200 should be replaced by a solubility test as the former is not relevant. Milk of magnesia is a suspension of solid magnesium hydroxide. The phosphates crystallize from the solutions as hydrates and are separated by centrifugation.

With the capability of absorbing water and free-flowing trisodium citrate anhydrous can be used as a carrier in moisture-sensitive formulations by providing a longer shelf life for its. Preparation of Citrate Buffer pH 3062 To create 100ml of a 01M citrate buffer mix citric acid monohydrate and trisodium citrate dehydrate as given below. The phosphates crystallize from the solutions as hydrates and are separated by centrifugation. Citrate may also exert an anticoagulant effect via a so far unknown mechanism as restoration of calcium concentration does not fully reverse the effect of citrate 1. Citric Acid UP168781 1 Kg MW1921 Citric Acid ACS grade Biotech grade 673410 500 g MW1921 Citric Acid Trisodium Dihydrate 218830 1 Kg 218831 25 Kg MW2941 Citric Acid Trisodium Dihydrate Proteomics Grade 10853A 500 g 10853B 1 Kg.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Milk of magnesia is a suspension of solid magnesium hydroxide. Citrate may also exert an anticoagulant effect via a so far unknown mechanism as restoration of calcium concentration does not fully reverse the effect of citrate 1. Trisodium citrate dihydrate is a tribasic salt of citric acid. Drying conditions for trisodium citrate E 331 iii should also be adjusted to improve the reproducibility of the method. Trisodium phosphate TSP Na3PO4 is a useful cleaning agent but it must be handled with care because its solutions are quite caustic.

Citrate is a weak base and so reacts with hydrochloric acid in the stomach to raise the pH. 01M citric acid monohydrate C 6 H 8 O 7 H 2 O FW 21014 Solution B. 01M trisodium citrate dihydrate C. With the capability of absorbing water and free-flowing trisodium citrate anhydrous can be used as a carrier in moisture-sensitive formulations by providing a longer shelf life for its. Trisodium citrate dihydrate is a tribasic salt of citric acid.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is widely used in foods beverages and various technical applications mainly. With the capability of absorbing water and free-flowing trisodium citrate anhydrous can be used as a carrier in moisture-sensitive formulations by providing a longer shelf life for its. Citrate is a weak base and so reacts with hydrochloric acid in the stomach to raise the pH. If a solution of Na3PO4 is added to one containing a calcium salt such as CaCl2 a precipitate of calcium phosphate is formed. The phosphates crystallize from the solutions as hydrates and are separated by centrifugation.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title trisodium citrate dihydrate solubility by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.