Vapor pressure of 1 propanol
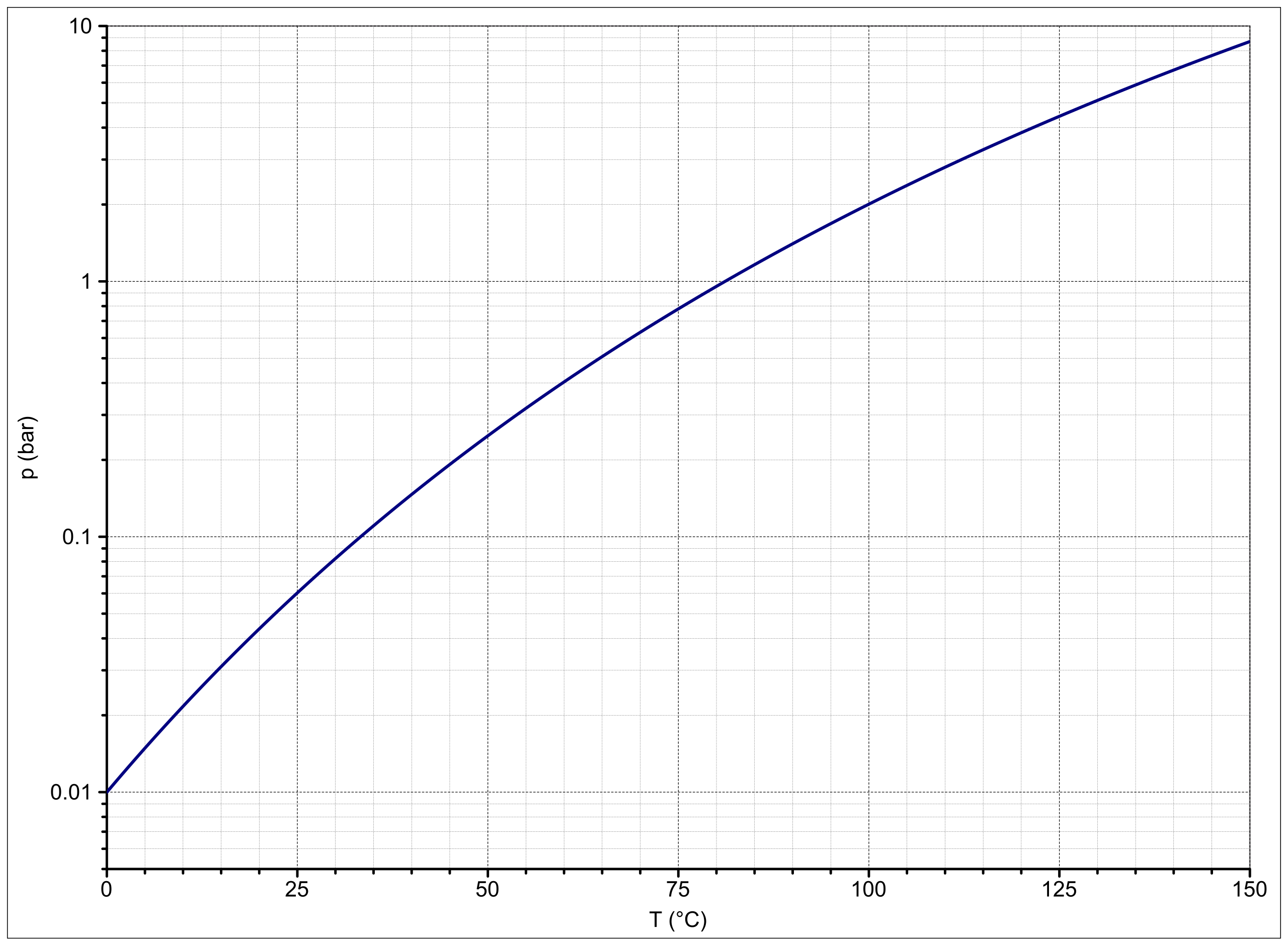
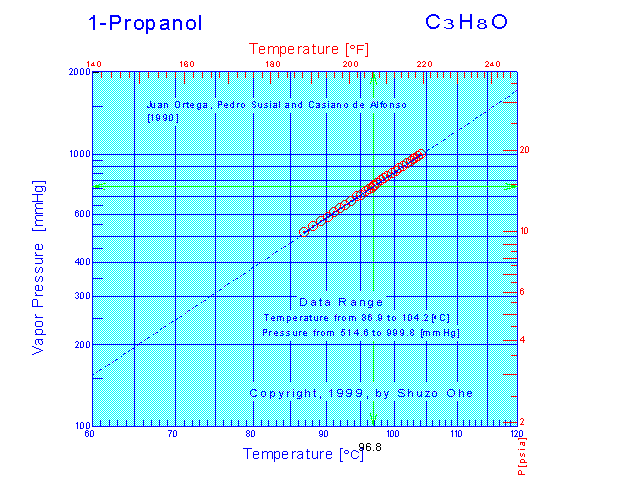
Vapor Pressure Of 1 Propanol. The vapor pressure of 1-propanol is 100 torr at 147 C. Ambrose and Townsend 1963 3. –ephedrine is a phenethylamine alkaloid that is 2-phenylethanamine substituted by a methyl group at the amino nitrogen and a methyl and a hydroxy group at position 2 and 1 respectively. To achieve precise design determination of vaporliqid equilibrium for the system is indispensable while maintaining efficacy and simplicity.

Calculate the vapor pressure at 528 C. It is formed by the esterification of acetic acid and 1-propanol known as a condensation reaction often via FischerSpeier esterification with sulfuric acid as a catalyst and water produced as a byproduct. This table gives azeotropic data for a number of binary mixtures at normal atmospheric pressure Paz 1013 kPa. Temperature K A B C Reference Comment. Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. Grant and contract funding is sourced from the US National Institutes of Health the Bill Melinda Gates Foundation The Wellcome Trust EDCTP the South African Medical Research Council the National Research Foundation of South.
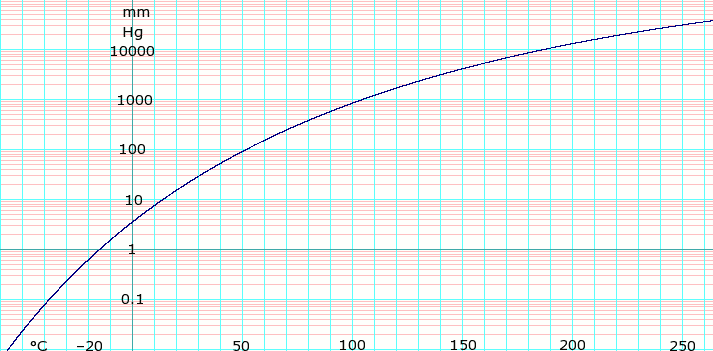
The vapor pressure is the pressure that the molecules at the surface of the liquid exert against the external pressure which is usually the atmospheric pressure.
It is produced on a large scale primarily as a precursor to the industrial solvent methyl ethyl ketone2-Butanol is chiral and thus can be obtained as either of two. Properties of Organic Solvents. The values in the table below except as noted have been extracted from online and hardbound compilations. Azeotropic point are the azeotropic temperature taz pressure Paz and liquid-phase composition usually expressed as mole fractions. Less dense than water. Heat of vaporization of 1-propanol 472 kJmol Solution.
 Source: ddbst.com
Source: ddbst.com
The vapor pressure of a substance roughly doubles for every increase in 10 C. Less dense than water. Calculate the vapor pressure at 528 C. The vapor pressure is a very sensitive function of temperature. Properties of Organic Solvents.
 Source: semanticscholar.org
Source: semanticscholar.org
–ephedrine is a phenethylamine alkaloid that is 2-phenylethanamine substituted by a methyl group at the amino nitrogen and a methyl and a hydroxy group at position 2 and 1 respectively. Calculate the vapor pressure at 528 C. It has a role as a nasal decongestant a sympathomimetic agent a vasoconstrictor agent a xenobiotic an environmental contaminant a plant metabolite and a bacterial metabolite. The vapor pressure is a very sensitive function of temperature. It is produced on a large scale primarily as a precursor to the industrial solvent methyl ethyl ketone2-Butanol is chiral and thus can be obtained as either of two.
 Source: commons.wikimedia.org
Source: commons.wikimedia.org
Heat of vaporization of 1-propanol 472 kJmol Solution. The vapor pressure is a very sensitive function of temperature. Ambrose and Townsend 1963 3. The vapor pressure of 1-propanol is 100 torr at 147 C. The Clausius-Clapeyron equation relates a solutions vapor pressures at different temperatures to the heat of vaporization.
 Source: chart-studio.plotly.com
Source: chart-studio.plotly.com
Temperature K A B C Reference Comment. It has a role as a nasal decongestant a sympathomimetic agent a vasoconstrictor agent a xenobiotic an environmental contaminant a plant metabolite and a bacterial metabolite. Flash point 85 - 100F. Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from. This study presents a simple model for estimating hydrogen sulfide solubility in aqueous alkanolamines and their blends in the high pressure-high gas loading region.
 Source: s-ohe.com
Source: s-ohe.com
Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. Less dense than water. The Clausius-Clapeyron equation relates a solutions vapor pressures at different temperatures to the heat of vaporization. The Clausius-Clapeyron equation is expressed by lnP T1vap P T2vap ΔH vap R1T 2 - 1T 1. 25 mmHg 20 C.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It does not increase linearly but in fact increases exponentially with temperature. Coefficents calculated by NIST from authors data. This table gives azeotropic data for a number of binary mixtures at normal atmospheric pressure Paz 1013 kPa. Calculate the vapor pressure at 528 C. Research in the IDM is led by over 34 independent principal investigators in the basic clinical and public health sciences and has a strong translational focus.
 Source: researchgate.net
Source: researchgate.net
To achieve precise design determination of vaporliqid equilibrium for the system is indispensable while maintaining efficacy and simplicity. It does not increase linearly but in fact increases exponentially with temperature. Isobutanol appears as a clear colorless liquid with a sweet odor. This table gives azeotropic data for a number of binary mixtures at normal atmospheric pressure Paz 1013 kPa. Isobutanol is an alkyl alcohol that is propan-1-ol substituted by a methyl.

The vapor pressure is the pressure that the molecules at the surface of the liquid exert against the external pressure which is usually the atmospheric pressure. Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from. Grant and contract funding is sourced from the US National Institutes of Health the Bill Melinda Gates Foundation The Wellcome Trust EDCTP the South African Medical Research Council the National Research Foundation of South. Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. Research in the IDM is led by over 34 independent principal investigators in the basic clinical and public health sciences and has a strong translational focus.
 Source: researchgate.net
Source: researchgate.net
Heat of vaporization of 1-propanol 472 kJmol Solution. –ephedrine is a phenethylamine alkaloid that is 2-phenylethanamine substituted by a methyl group at the amino nitrogen and a methyl and a hydroxy group at position 2 and 1 respectively. Isobutanol appears as a clear colorless liquid with a sweet odor. Isobutanol is an alkyl alcohol that is propan-1-ol substituted by a methyl. Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from.
 Source: semanticscholar.org
Source: semanticscholar.org
Vapors heavier than air. 25 mmHg 20 C. This study presents a simple model for estimating hydrogen sulfide solubility in aqueous alkanolamines and their blends in the high pressure-high gas loading region. Flash point 85 - 100F. It is produced on a large scale primarily as a precursor to the industrial solvent methyl ethyl ketone2-Butanol is chiral and thus can be obtained as either of two.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title vapor pressure of 1 propanol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.