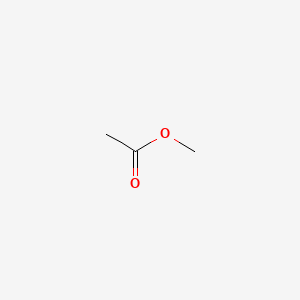
Vapor pressure of n octane
Vapor Pressure Of N Octane. Direct photolysis is not expected to be an important. Hydrogen - Thermophysical Properties - Chemical Physical and Thermal Properties of Hydrogen - H 2. The half-life for this reaction in air is estimated to be 23 days. N-Octane 11422 Nitrous Oxide N 2O 44013 0114 Nitrous Trioxide NO 3 62005 Oxygen O 2 32 1331 1 14292 00831 008922 Ozone O 3 480 0125 N-Pentane 7215 Gas Formula Molecular weight Density - ρ-kgm 3lb m ft3 Formula Density - ρ 3 ft3 1 NTP - Normal Temperature and Pressure - is defined as air at 20 oC 29315 K 68 F and 1 atm.
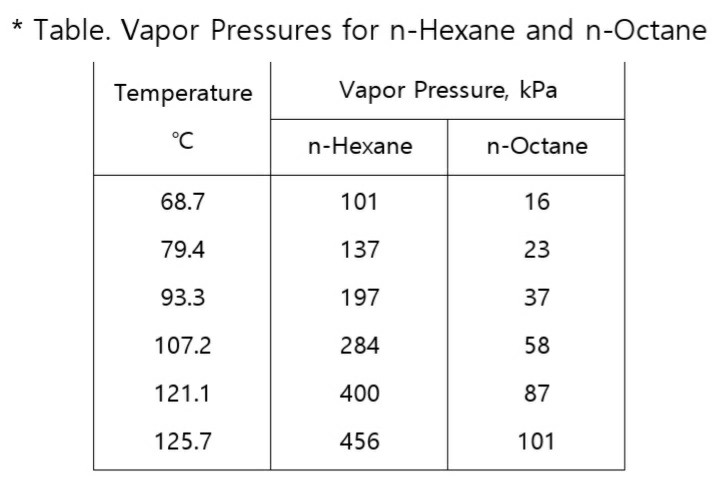
 Oneclass For Problems 2 And 3 Use The N Hexane N Octane Data From Problem 1 Number 1 Answer Is Sh From oneclass.com
Oneclass For Problems 2 And 3 Use The N Hexane N Octane Data From Problem 1 Number 1 Answer Is Sh From oneclass.com
B and C only 3. One of these isomers 224-trimethylpentane commonly called iso-octane is used as one of the standard values in the octane rating scale. It was first proposed by Kamerlingh Onnes. The half-life for this reaction in air is estimated to be 23 days. Classic equation for vapor pressure calculation and correlation. Draw the Lewis.
A and C only 5.
A and C only 5. It was first proposed by Kamerlingh Onnes. A and B only. None of the properties 7. Direct photolysis is not expected to be an important. The compressibility factor is a.
 Source: researchgate.net
Source: researchgate.net
Vapor Pressure 25C mm Hg kPa Density 25C gmL cycl ohexane hexahydrobenzene CAS 110-82-7 RTECS GU6300000 C 6 H 12 8416 807 976 130 0779 cycl ohexene tetrahydrobenzene CAS 110-83-8 RTECS GW2500000 C 6 H 10 8215 830 888 118 0811 n-decane CAS 124-18-5 RTECS HD6550000 C 10 H 22 14228 174 NA NA 0730 n-dodecane CAS. N-Octane 11422 Nitrous Oxide N 2O 44013 0114 Nitrous Trioxide NO 3 62005 Oxygen O 2 32 1331 1 14292 00831 008922 Ozone O 3 480 0125 N-Pentane 7215 Gas Formula Molecular weight Density - ρ-kgm 3lb m ft3 Formula Density - ρ 3 ft3 1 NTP - Normal Temperature and Pressure - is defined as air at 20 oC 29315 K 68 F and 1 atm. If released to air a vapor pressure of 1493 mm Hg at 25 C indicates ethyl methyl ether will exist solely as a vapor in the ambient atmosphere. Hydrogen - Thermophysical Properties - Chemical Physical and Thermal Properties of Hydrogen - H 2. A B and C 8.
 Source: chegg.com
Source: chegg.com
This is the virial equation of state the most general function relating pressure P density ρ and temperature T of fluids. Octane is a hydrocarbon and an alkane with the chemical formula C 8 H 18 and the condensed structural formula CH 3 CH 2 6 CH 3Octane has many structural isomers that differ by the amount and location of branching in the carbon chain. Vapor Pressure 25C mm Hg kPa Density 25C gmL cycl ohexane hexahydrobenzene CAS 110-82-7 RTECS GU6300000 C 6 H 12 8416 807 976 130 0779 cycl ohexene tetrahydrobenzene CAS 110-83-8 RTECS GW2500000 C 6 H 10 8215 830 888 118 0811 n-decane CAS 124-18-5 RTECS HD6550000 C 10 H 22 14228 174 NA NA 0730 n-dodecane CAS. One of these isomers 224-trimethylpentane commonly called iso-octane is used as one of the standard values in the octane rating scale. The high boiling point is due to a lower vapor pressure.
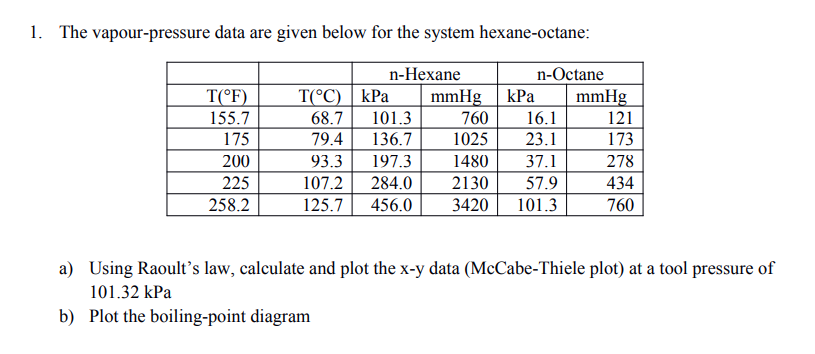
 Source: oneclass.com
Source: oneclass.com
If released to air a vapor pressure of 1493 mm Hg at 25 C indicates ethyl methyl ether will exist solely as a vapor in the ambient atmosphere. Vapor Pressure 25C mm Hg kPa Density 25C gmL cycl ohexane hexahydrobenzene CAS 110-82-7 RTECS GU6300000 C 6 H 12 8416 807 976 130 0779 cycl ohexene tetrahydrobenzene CAS 110-83-8 RTECS GW2500000 C 6 H 10 8215 830 888 118 0811 n-decane CAS 124-18-5 RTECS HD6550000 C 10 H 22 14228 174 NA NA 0730 n-dodecane CAS. This is the virial equation of state the most general function relating pressure P density ρ and temperature T of fluids. B and C only 3. It was first proposed by Kamerlingh Onnes.
 Source: chegg.com
Source: chegg.com
Octane is a hydrocarbon and an alkane with the chemical formula C 8 H 18 and the condensed structural formula CH 3 CH 2 6 CH 3Octane has many structural isomers that differ by the amount and location of branching in the carbon chain. B and C only correct The stronger intermolecular forces will lead to higher viscosity and a higher boiling point. Vapor Pressure of n-Heptane Temperature C Vapor Pressure mm Hg -340 1 -21 10 223 40 418 100 780 400 984 760 1248 1520 1657 3800 2028 7600 2475 15200 3190 30400 2-21. One of these isomers 224-trimethylpentane commonly called iso-octane is used as one of the standard values in the octane rating scale. The compressibility factor is a.
 Source: chegg.com
Source: chegg.com
Direct photolysis is not expected to be an important. A B and C 8. A higher boiling point. You want to calculate. Vapor Pressure 25C mm Hg kPa Density 25C gmL cycl ohexane hexahydrobenzene CAS 110-82-7 RTECS GU6300000 C 6 H 12 8416 807 976 130 0779 cycl ohexene tetrahydrobenzene CAS 110-83-8 RTECS GW2500000 C 6 H 10 8215 830 888 118 0811 n-decane CAS 124-18-5 RTECS HD6550000 C 10 H 22 14228 174 NA NA 0730 n-dodecane CAS.
 Source: oneclass.com
Source: oneclass.com
A and C only 5. None of the properties 7. A B and C 8. B and C only 3. You want to calculate.
 Source: thermopedia.com
Source: thermopedia.com
1 NTP - Normal Temperature and Pressure - is defined as 20 o C 29315 K 68 o F and 1 atm 101325 kNm2 101325 kPa 147 psia 0 psig 30 in Hg 760 torr Since specific gravity is the ratio between the density mass per unit volume of an actual gas and the density of air - specific gravity has no dimension. Where is the compressibility factor. A and B only. A B and C 8. One of these isomers 224-trimethylpentane commonly called iso-octane is used as one of the standard values in the octane rating scale.
 Source: sciencedirect.com
Source: sciencedirect.com
Vapor-phase ethyl methyl ether will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals. None of the properties 7. Melting point from molecular weight - Calculation of melting point of hydrocarbons from molecular weight molar mass. A and B only. One of these isomers 224-trimethylpentane commonly called iso-octane is used as one of the standard values in the octane rating scale.
 Source: researchgate.net
Source: researchgate.net
Vapor Pressure 25C mm Hg kPa Density 25C gmL cycl ohexane hexahydrobenzene CAS 110-82-7 RTECS GU6300000 C 6 H 12 8416 807 976 130 0779 cycl ohexene tetrahydrobenzene CAS 110-83-8 RTECS GW2500000 C 6 H 10 8215 830 888 118 0811 n-decane CAS 124-18-5 RTECS HD6550000 C 10 H 22 14228 174 NA NA 0730 n-dodecane CAS. Vapor-phase ethyl methyl ether will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals. The vapor pressure is given in the following table. This is the virial equation of state the most general function relating pressure P density ρ and temperature T of fluids. Hydrogen - Thermophysical Properties - Chemical Physical and Thermal Properties of Hydrogen - H 2.
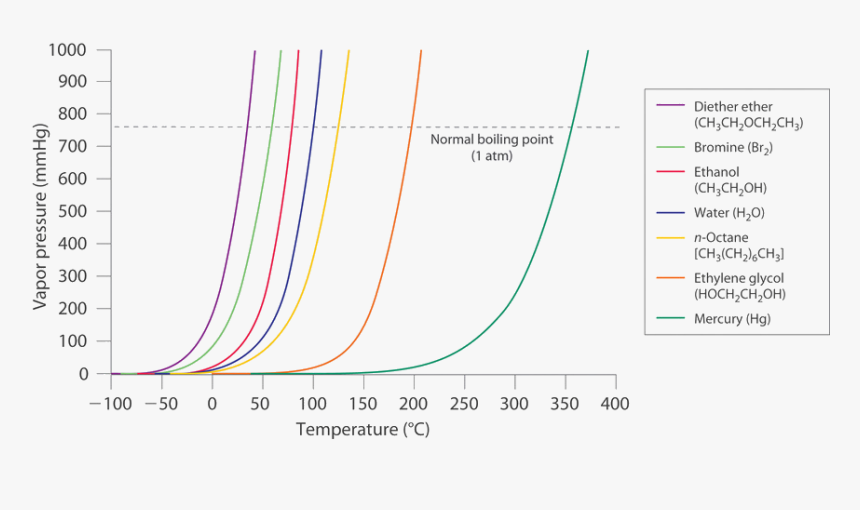 Source: kindpng.com
Source: kindpng.com
Vapor-phase ethyl methyl ether will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals. Direct photolysis is not expected to be an important. A B and C 8. Vapor-phase ethyl methyl ether will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals. A and B only.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title vapor pressure of n octane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.