Vinyl chloride copolymers
Vinyl Chloride Copolymers. 1772000 - Vinylidene chloridemethyl acrylatemethyl methacrylate. D5260 Classification for Chemical Resistance of PolyVinyl Chloride PVC Homopolymer and Copolymer Compounds and Chlorinated PolyVinyl Chloride CPVC Compounds. 1771950 - Vinyl chloride-ethylene copolymers. Vinyl acetate was once prepared by.
 Polyvinyl Chloride Acetate Wikipedia From en.wikipedia.org
Polyvinyl Chloride Acetate Wikipedia From en.wikipedia.org
At that time the material was reported to be an off-white solid that could be heated to 130 degrees C without degradation. Polymers or copolymers of vinyl chloride PVC 3904 10 3904 21 polyurethane PUR 3909 50 low-density polyethylene LDPE with the exception of low -density polyethylene used for the production of coloured masterbatch 3901 10 cellulose acetate CA 3912 11 cellulose acetate butyrate CAB 3912 11 epoxy resins 3907 30 melamine-formaldehyde MF resins. 1771950 - Vinyl chloride-ethylene copolymers. 1771990 - Vinylidene chloridemethyl acrylate copolymers. ISO 1163-11985 PlasticsUnplasticized compounds of homopolymers and copolymers of. PVC was discovered as early as 1835 but the first definite report of the polymerization of vinyl chloride did not come until about 35 years later.
The vinylidene chloride copolymers.
The vinyl chloride copolymers shall contain not less than 50 weight percent of total polymer units derived from vinyl chloride. Vinyl chloride is primarily used to make polyvinyl chloride to manufacture plastics. 1772000 - Vinylidene chloridemethyl acrylatemethyl methacrylate. The vinylidene chloride copolymers. The vinyl chloride copolymers shall contain not less than 50 weight percent of total polymer units derived from vinyl chloride. Polymers or copolymers of vinyl chloride PVC 3904 10 3904 21 polyurethane PUR 3909 50 low-density polyethylene LDPE with the exception of low -density polyethylene used for the production of coloured masterbatch 3901 10 cellulose acetate CA 3912 11 cellulose acetate butyrate CAB 3912 11 epoxy resins 3907 30 melamine-formaldehyde MF resins.
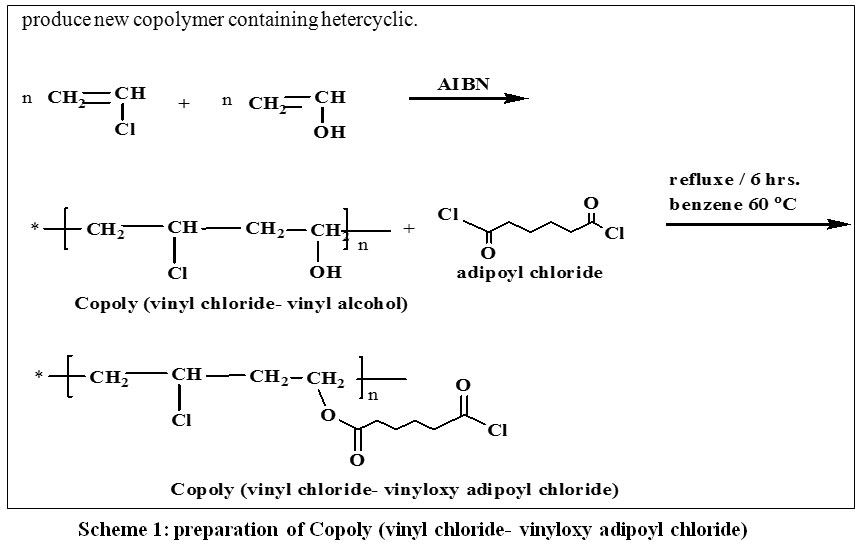 Source: orientjchem.org
Source: orientjchem.org
PVC was discovered as early as 1835 but the first definite report of the polymerization of vinyl chloride did not come until about 35 years later. Vinyl Chloride is a chlorinated hydrocarbon occurring as a colorless highly flammable gas with a mild sweet odor that may emit toxic fumes of carbon dioxide carbon monoxide hydrogen chloride and phosgene when heated to decomposition. 1772000 - Vinylidene chloridemethyl acrylatemethyl methacrylate. PVC remained a laboratory curiosity for many years probably because of its intractable nature. The vinylidene chloride copolymers.
 Source: britannica.com
Source: britannica.com
1772000 - Vinylidene chloridemethyl acrylatemethyl methacrylate. 1771960 - Vinyl chloride-hexene-1 copolymers. 1771990 - Vinylidene chloridemethyl acrylate copolymers. 1772000 - Vinylidene chloridemethyl acrylatemethyl methacrylate. PVC was discovered as early as 1835 but the first definite report of the polymerization of vinyl chloride did not come until about 35 years later.
 Source: en.wikipedia.org
Source: en.wikipedia.org
1771960 - Vinyl chloride-hexene-1 copolymers. 1771990 - Vinylidene chloridemethyl acrylate copolymers. 1771950 - Vinyl chloride-ethylene copolymers. Vinyl chloride is primarily used to make polyvinyl chloride to manufacture plastics. ISO 1163-11985 PlasticsUnplasticized compounds of homopolymers and copolymers of.
 Source: chemicalbook.com
Source: chemicalbook.com
Polyvinylchloride -co -vinylidene chloride Saran A -B -A -B -A -B alternati ng copolymer A -A -A -A -B -A -B random copolymer Both of these are rare. ISO 1163-11985 PlasticsUnplasticized compounds of homopolymers and copolymers of. PVC was discovered as early as 1835 but the first definite report of the polymerization of vinyl chloride did not come until about 35 years later. D5260 Classification for Chemical Resistance of PolyVinyl Chloride PVC Homopolymer and Copolymer Compounds and Chlorinated PolyVinyl Chloride CPVC Compounds. Vinyl acetate was once prepared by.
 Source: shinetsu.co.jp
Source: shinetsu.co.jp
Due to the instability of the radical attempts to control the polymerization by most livingcontrolled radical. The vinylidene chloride copolymers. 1771950 - Vinyl chloride-ethylene copolymers. There are three different types of EVA copolymer which differ in the vinyl acetate VA content and the way the materials are used. Due to the instability of the radical attempts to control the polymerization by most livingcontrolled radical.
 Source: pg-chem.com
Source: pg-chem.com
The vinyl chloride copolymers shall contain not less than 50 weight percent of total polymer units derived from vinyl chloride. Block copolymers Two long sequences of repeat units. PVC remained a laboratory curiosity for many years probably because of its intractable nature. 1772000 - Vinylidene chloridemethyl acrylatemethyl methacrylate. There are three different types of EVA copolymer which differ in the vinyl acetate VA content and the way the materials are used.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Ethylene-vinyl acetate EVA also known as poly ethylene-vinyl acetate PEVA is the copolymer of ethylene and vinyl acetateThe weight percent of vinyl acetate usually varies from 10 to 40 with the remainder being ethylene. At levels not to exceed 01 percent by weight of vinylidene chloride homopolymers andor vinylidene chloride copolymers used in accordance with a prior sanction or applicable regulations in parts 175 176 and 177 of this chapter. Vinyl Chloride is a chlorinated hydrocarbon occurring as a colorless highly flammable gas with a mild sweet odor that may emit toxic fumes of carbon dioxide carbon monoxide hydrogen chloride and phosgene when heated to decomposition. Vinylidene chloride H2CCCl2 or C2H2Cl2 CID 6366 - structure chemical names physical and chemical properties classification patents literature biological. The polymer was inert to.
 Source: pvc.kaneka.co.jp
Source: pvc.kaneka.co.jp
Vinyl Chloride is a chlorinated hydrocarbon occurring as a colorless highly flammable gas with a mild sweet odor that may emit toxic fumes of carbon dioxide carbon monoxide hydrogen chloride and phosgene when heated to decomposition. 1771950 - Vinyl chloride-ethylene copolymers. 1771980 - Vinyl chloride-propylene copolymers. 1772000 - Vinylidene chloridemethyl acrylatemethyl methacrylate. Ethylene-vinyl acetate EVA also known as poly ethylene-vinyl acetate PEVA is the copolymer of ethylene and vinyl acetateThe weight percent of vinyl acetate usually varies from 10 to 40 with the remainder being ethylene.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
1772000 - Vinylidene chloridemethyl acrylatemethyl methacrylate. There are three different types of EVA copolymer which differ in the vinyl acetate VA content and the way the materials are used. At that time the material was reported to be an off-white solid that could be heated to 130 degrees C without degradation. Vinyl acetate was once prepared by. 1772000 - Vinylidene chloridemethyl acrylatemethyl methacrylate.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Block copolymers Two long sequences of repeat units. There are three different types of EVA copolymer which differ in the vinyl acetate VA content and the way the materials are used. Vinyl acetate was once prepared by. Vinylidene chloride H2CCCl2 or C2H2Cl2 CID 6366 - structure chemical names physical and chemical properties classification patents literature biological. 1771970 - Vinyl chloride-lauryl vinyl ether copolymers.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title vinyl chloride copolymers by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.