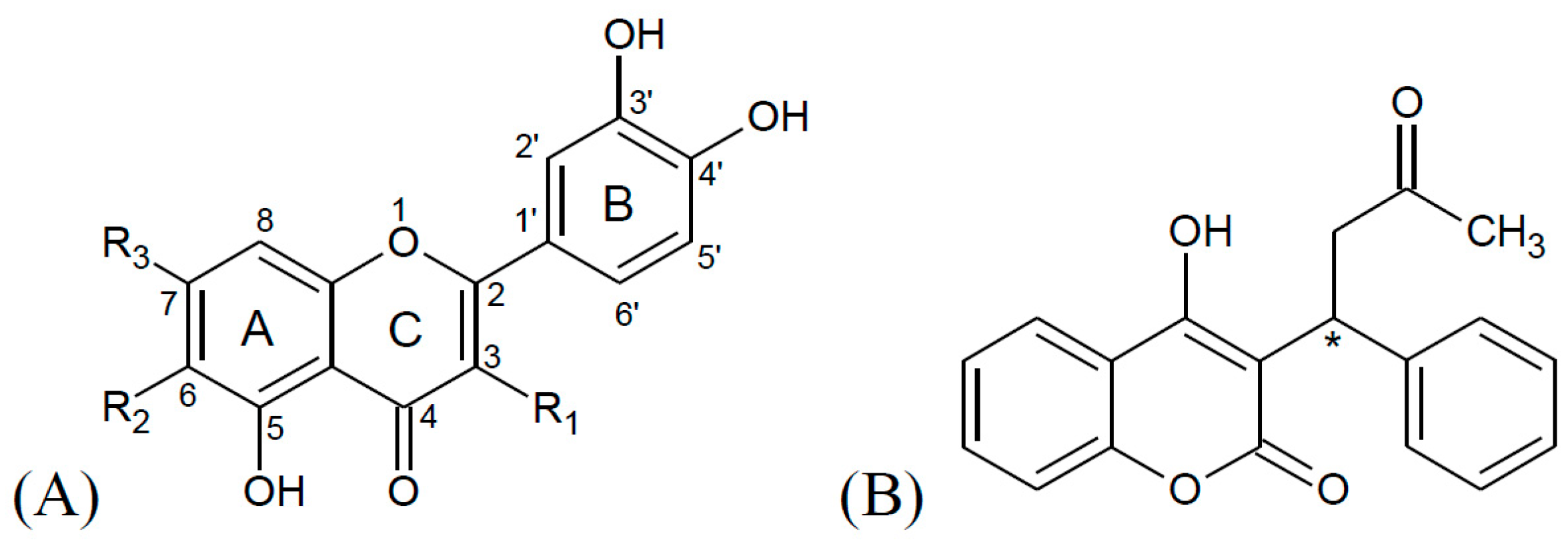
Warfarin structure physiological ph
Warfarin Structure Physiological Ph. Phenolate ion at physiological pH 25 BZ and DMBZ are more likely to be uncharged. Losartan irbesartan olmesartan candesartan valsartan fimasartan and azilsartan include the tetrazole group a ring with four nitrogen and one carbon. Synthesis of BBR metabolites and other derivatives has Two separate series of compounds allow us to assess the been previously reported 17. L Baardsnes J Kuiper M.

Identification of the ice-binding. B 357 927935 2002. Losartan irbesartan olmesartan candesartan and telmisartan include one or two imidazole groups. Structure and function of antifreeze proteins. The changes in urinary pH however assume practical importance only if the pKa of the drug ie the pH at which 50 of the molecules in solution is present in ionized form is between 75 and 105 for the bases and between 3. Enzymatic assays were effect.
Structure and function of antifreeze proteins.
The increased affinities of the halogenated analogs are consistent with the phenol group Results binding within an anionic pocket of EYA3. Enzymatic assays were effect. In particular if the pH of the urine is alkaline the absorption of acidic drugs is reduced while in the presence of an acidic pH basic drug absorption is reduced. B 357 927935 2002. Synthesis of BBR metabolites and other derivatives has Two separate series of compounds allow us to assess the been previously reported 17. The changes in urinary pH however assume practical importance only if the pKa of the drug ie the pH at which 50 of the molecules in solution is present in ionized form is between 75 and 105 for the bases and between 3.
 Source: jbc.org
Source: jbc.org
Losartan irbesartan olmesartan candesartan and telmisartan include one or two imidazole groups. Enzymatic assays were effect. Synthesis of BBR metabolites and other derivatives has Two separate series of compounds allow us to assess the been previously reported 17. The changes in urinary pH however assume practical importance only if the pKa of the drug ie the pH at which 50 of the molecules in solution is present in ionized form is between 75 and 105 for the bases and between 3. Phenolate ion at physiological pH 25 BZ and DMBZ are more likely to be uncharged.
 Source: mdpi.com
Source: mdpi.com
Losartan irbesartan olmesartan candesartan and telmisartan include one or two imidazole groups. Losartan irbesartan olmesartan candesartan valsartan fimasartan and azilsartan include the tetrazole group a ring with four nitrogen and one carbon. Losartan irbesartan olmesartan candesartan and telmisartan include one or two imidazole groups. Identification of the ice-binding. Enzymatic assays were effect.
 Source: jpharmsci.org
Source: jpharmsci.org
The increased affinities of the halogenated analogs are consistent with the phenol group Results binding within an anionic pocket of EYA3. Phenolate ion at physiological pH 25 BZ and DMBZ are more likely to be uncharged. Enzymatic assays were effect. These substances are AT 1-receptor antagonists. That is they block the activation of angiotensin II AT 1.
 Source: sciencedirect.com
Source: sciencedirect.com
These substances are AT 1-receptor antagonists. Identification of the ice-binding. B 357 927935 2002. Structure and function of antifreeze proteins. Crystals of the space group P3121 with a unit-cell dimension of a ¼ 515 Å b ¼ 515 Å and 19.

Synthesis of BBR metabolites and other derivatives has Two separate series of compounds allow us to assess the been previously reported 17. Phenolate ion at physiological pH 25 BZ and DMBZ are more likely to be uncharged. Losartan irbesartan olmesartan candesartan valsartan fimasartan and azilsartan include the tetrazole group a ring with four nitrogen and one carbon. Synthesis of BBR metabolites and other derivatives has Two separate series of compounds allow us to assess the been previously reported 17. The changes in urinary pH however assume practical importance only if the pKa of the drug ie the pH at which 50 of the molecules in solution is present in ionized form is between 75 and 105 for the bases and between 3.
 Source: researchgate.net
Source: researchgate.net
These substances are AT 1-receptor antagonists. The increased affinities of the halogenated analogs are consistent with the phenol group Results binding within an anionic pocket of EYA3. That is they block the activation of angiotensin II AT 1. B 357 927935 2002. Losartan irbesartan olmesartan candesartan valsartan fimasartan and azilsartan include the tetrazole group a ring with four nitrogen and one carbon.

The increased affinities of the halogenated analogs are consistent with the phenol group Results binding within an anionic pocket of EYA3. In particular if the pH of the urine is alkaline the absorption of acidic drugs is reduced while in the presence of an acidic pH basic drug absorption is reduced. Synthesis of BBR metabolites and other derivatives has Two separate series of compounds allow us to assess the been previously reported 17. Enzymatic assays were effect. The increased affinities of the halogenated analogs are consistent with the phenol group Results binding within an anionic pocket of EYA3.
 Source: sciencedirect.com
Source: sciencedirect.com
These substances are AT 1-receptor antagonists. That is they block the activation of angiotensin II AT 1. The changes in urinary pH however assume practical importance only if the pKa of the drug ie the pH at which 50 of the molecules in solution is present in ionized form is between 75 and 105 for the bases and between 3. These substances are AT 1-receptor antagonists. Enzymatic assays were effect.

L Baardsnes J Kuiper M. The increased affinities of the halogenated analogs are consistent with the phenol group Results binding within an anionic pocket of EYA3. Losartan irbesartan olmesartan candesartan and telmisartan include one or two imidazole groups. That is they block the activation of angiotensin II AT 1. The changes in urinary pH however assume practical importance only if the pKa of the drug ie the pH at which 50 of the molecules in solution is present in ionized form is between 75 and 105 for the bases and between 3.
 Source: dmd.aspetjournals.org
Source: dmd.aspetjournals.org
Synthesis of BBR metabolites and other derivatives has Two separate series of compounds allow us to assess the been previously reported 17. The increased affinities of the halogenated analogs are consistent with the phenol group Results binding within an anionic pocket of EYA3. Enzymatic assays were effect. Identification of the ice-binding. That is they block the activation of angiotensin II AT 1.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title warfarin structure physiological ph by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.