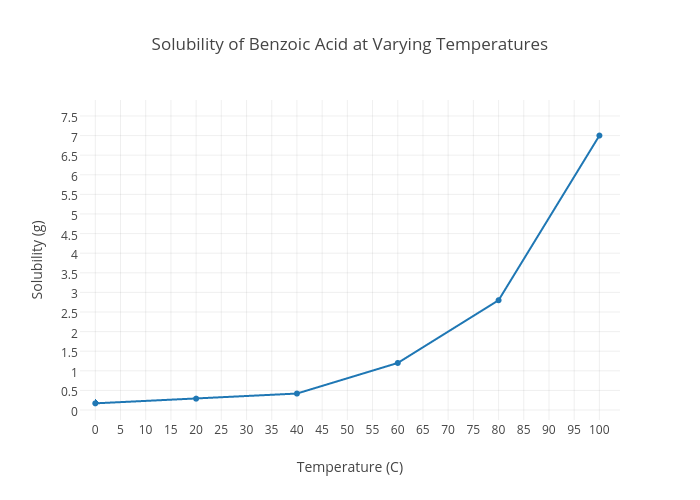
Water solubility benzoic acid
Water Solubility Benzoic Acid. It has a role as a toxin a human metabolite an Escherichia coli metabolite a plant metabolite a Saccharomyces cerevisiae metabolite an EC 6411 pyruvate carboxylase inhibitor an Aspergillus metabolite a plant growth retardant an allergen. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. 122 ºC the eutectic point is 82 ºC. Again hydroxyl compounds are listed on the left.
 Effect Of Nacl Concentration On The Solubility Of Benzoic Acid In Water Download Table From researchgate.net
Effect Of Nacl Concentration On The Solubility Of Benzoic Acid In Water Download Table From researchgate.net
It is a white crystalline compound that is slightly soluble in water and freely soluble in many organic solvents. Again hydroxyl compounds are listed on the left. 137 ºC and B is benzoic acid mp. Cinnamic acid is an organic compound with the formula C 6 H 5 CHCHCOOH. The pH of solutions of carboxylic acids is therefore less than 70. To 01 by weight.
Phenylacetic acid is a monocarboxylic acid that is toluene in which one of the hydrogens of the methyl group has been replaced by a carboxy group.
CHEM 2423 Recrystallization of Benzoic Acid Dr. 122 ºC the eutectic point is 82 ºC. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Benzoic acid is generally not used directly due to its poor water solubility. 7 Sodium benzoate is also allowed as an animal food additive at up to 01 per the Association of American Feed Control Officials. A second oxygen atom dramatically increases water solubility as demonstrated by the compounds listed in the third row.
 Source: ochemonline.wordpress.com
Source: ochemonline.wordpress.com
Classified as an unsaturated carboxylic acid it occurs naturally in a number of plantsIt exists as both a cis and a trans isomer although the latter is more common. Phenylacetic acid is a monocarboxylic acid that is toluene in which one of the hydrogens of the methyl group has been replaced by a carboxy group. For example if A is cinnamic acid mp. Cinnamic acid is an organic compound with the formula C 6 H 5 CHCHCOOH. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with.
 Source: researchgate.net
Source: researchgate.net
Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 70. Benzoic acid is generally not used directly due to its poor water solubility. Again hydroxyl compounds are listed on the left.

Phenylacetic acid is a monocarboxylic acid that is toluene in which one of the hydrogens of the methyl group has been replaced by a carboxy group. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. 7 Sodium benzoate is also allowed as an animal food additive at up to 01 per the Association of American Feed Control Officials. The pH of solutions of carboxylic acids is therefore less than 70. It has a role as a toxin a human metabolite an Escherichia coli metabolite a plant metabolite a Saccharomyces cerevisiae metabolite an EC 6411 pyruvate carboxylase inhibitor an Aspergillus metabolite a plant growth retardant an allergen.
 Source: degruyter.com
Source: degruyter.com
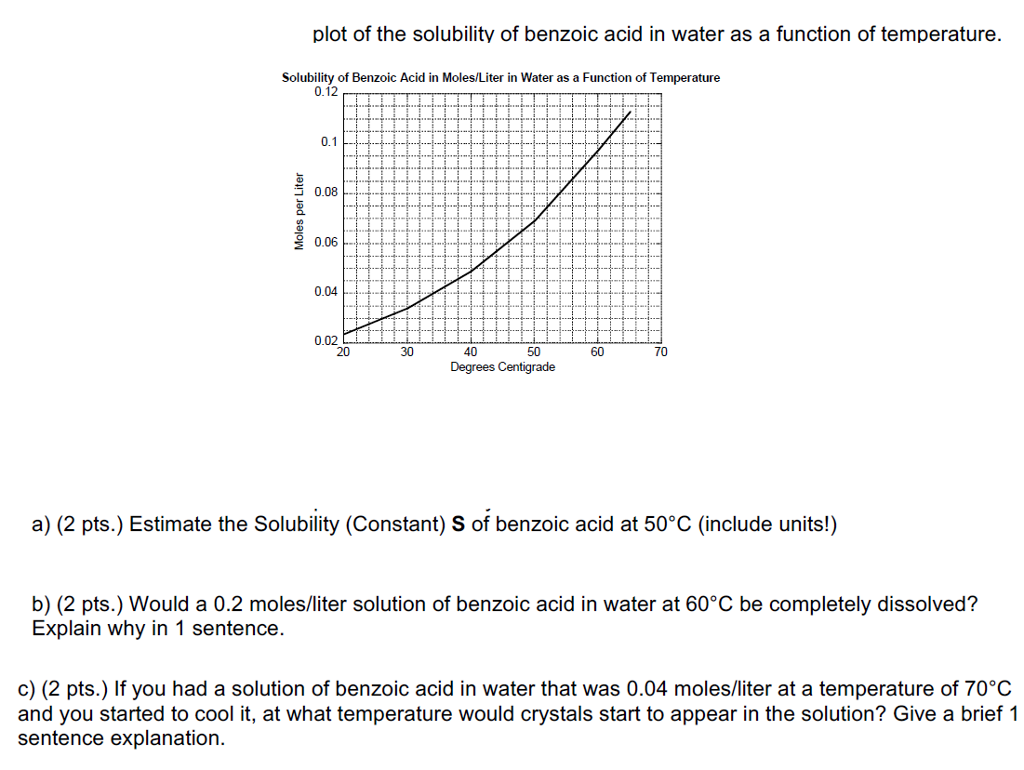
Phenylacetic acid is a monocarboxylic acid that is toluene in which one of the hydrogens of the methyl group has been replaced by a carboxy group. It has a role as a toxin a human metabolite an Escherichia coli metabolite a plant metabolite a Saccharomyces cerevisiae metabolite an EC 6411 pyruvate carboxylase inhibitor an Aspergillus metabolite a plant growth retardant an allergen. 137 ºC and B is benzoic acid mp. Pahlavan 3 Example 1- The solubility of solid X in hot water 550 g100 ml at 100 o C is not very great and its solubility in cold water 053 g100ml at 0 o C is significant. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions.
 Source: sciencedirect.com
Source: sciencedirect.com
7 Sodium benzoate is also allowed as an animal food additive at up to 01 per the Association of American Feed Control Officials. A second oxygen atom dramatically increases water solubility as demonstrated by the compounds listed in the third row. Benzoic acid is generally not used directly due to its poor water solubility. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. To 01 by weight.
 Source: sciencedirect.com
Source: sciencedirect.com
It has a role as a toxin a human metabolite an Escherichia coli metabolite a plant metabolite a Saccharomyces cerevisiae metabolite an EC 6411 pyruvate carboxylase inhibitor an Aspergillus metabolite a plant growth retardant an allergen. 122 ºC the eutectic point is 82 ºC. For example if A is cinnamic acid mp. Assuming the solution is chilled at 0. Concentration as a food preservative is limited by the FDA in the US.
 Source: chegg.com
Source: chegg.com
What would be the maximum theoretical percent recovery from crystallization of 500 g of solid X from 100 ml water. Assuming the solution is chilled at 0. Cinnamic acid is an organic compound with the formula C 6 H 5 CHCHCOOH. 7 Sodium benzoate is also allowed as an animal food additive at up to 01 per the Association of American Feed Control Officials. Benzoic acid is generally not used directly due to its poor water solubility.
 Source: researchgate.net
Source: researchgate.net
It has a role as a toxin a human metabolite an Escherichia coli metabolite a plant metabolite a Saccharomyces cerevisiae metabolite an EC 6411 pyruvate carboxylase inhibitor an Aspergillus metabolite a plant growth retardant an allergen. 122 ºC the eutectic point is 82 ºC. The pH of solutions of carboxylic acids is therefore less than 70. Nitrogen exerts a solubilizing influence similar to oxygen as shown by the compounds in the fourth row. 137 ºC and B is benzoic acid mp.
 Source: chart-studio.plotly.com
Source: chart-studio.plotly.com
Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. To 01 by weight. Benzoic acid is generally not used directly due to its poor water solubility. Cinnamic acid is an organic compound with the formula C 6 H 5 CHCHCOOH.
 Source: degruyter.com
Source: degruyter.com
CHEM 2423 Recrystallization of Benzoic Acid Dr. Again hydroxyl compounds are listed on the left. Cinnamic acid is an organic compound with the formula C 6 H 5 CHCHCOOH. Nitrogen exerts a solubilizing influence similar to oxygen as shown by the compounds in the fourth row. Benzoic acid is generally not used directly due to its poor water solubility.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title water solubility benzoic acid by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.