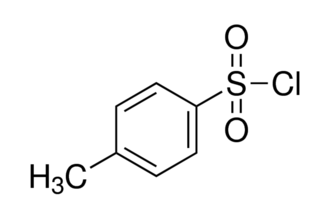
What is dimethyl ether formula
What Is Dimethyl Ether Formula. The reaction occurs with inversion of configuration at chiral centers and can be limited by possible competing elimination reactions. Any leak can be either liquid or vapor. The Williamson ether synthesis is an S N 2 reaction in which an alkoxide ion is a nucleophile that displaces a halide ion from an alkyl halide to give an ether. Intramolecular Williamson ether synthesis occurs at rates that depend on the number of atoms in the.
 Dimethylether Gas Encyclopedia Air Liquide Air Liquide From encyclopedia.airliquide.com
Dimethylether Gas Encyclopedia Air Liquide Air Liquide From encyclopedia.airliquide.com
Dimethyl ether is a colorless gas with a faint ethereal odor. To do so the common alkoxy substituents are given names derived from their alkyl component below. The reaction occurs with inversion of configuration at chiral centers and can be limited by possible competing elimination reactions. C 6 H 14 O 3 or CH 3 CH 2 OCH 2 CH 2 OCH 2 CH 2 OH. The ether functional group does not have a characteristic IUPAC nomenclature suffix so it is necessary to designate it as a substituent. Dimethoxyethane is miscible with water.
Ethers are compounds having two alkyl or aryl groups bonded to an oxygen atom as in the formula R 1 OR 2.
Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. The reaction occurs with inversion of configuration at chiral centers and can be limited by possible competing elimination reactions. C 6 H 14 O 3 or CH 3 CH 2 OCH 2 CH 2 OCH 2 CH 2 OH. Ethers are compounds having two alkyl or aryl groups bonded to an oxygen atom as in the formula R 1 OR 2. Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. Flash point near 190F.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
Alkyl Group Name Alkoxy Group Name. The systematic name for the compound in Problem 21 is _____. Diethylene glycol monoethyl ether appears as a colorless slightly viscous liquid with a mild pleasant odor. The compound below is classified as a _____. The simplest ether it is a colorless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applicationsIt is an isomer of ethanol.
 Source: commons.wikimedia.org
Source: commons.wikimedia.org
Dimethyl ether is a colorless gas with a faint ethereal odor. Dimethoxyethane also known as glyme monoglyme dimethyl glycol ethylene glycol dimethyl ether dimethyl cellosolve and DME is a colorless aprotic and liquid ether that is used as a solvent especially in batteries. Alkyl Group Name Alkoxy Group Name. The Government of Canada has the authority to regulate and authorize other instruments to prevent or control the use andor release of these substances. A pentyl amine b methyl-n-propyl amine.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
To do so the common alkoxy substituents are given names derived from their alkyl component below. Intramolecular Williamson ether synthesis occurs at rates that depend on the number of atoms in the. Any leak can be either liquid or vapor. A pentyl amine b methyl-n-propyl amine. Dimethyl ether DME also known as methoxymethane is the organic compound with the formula CH 3 OCH 3 simplified to C 2 H 6 O.
 Source: molinstincts.com
Source: molinstincts.com
Diethylene glycol monoethyl ether. It can asphyxiate by the displacement of air. Diethylene glycol monoethyl ether appears as a colorless slightly viscous liquid with a mild pleasant odor. It is easily ignited. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Diethylene glycol monoethyl ether appears as a colorless slightly viscous liquid with a mild pleasant odor. The List of Toxic Substances in Schedule 1 of the Canadian Environmental Protection Act 1999 CEPA 1999 includes substances that are considered to be toxic as defined in Section 64 of the Act. Dimethyl ether DME also known as methoxymethane is the organic compound with the formula CH 3 OCH 3 simplified to C 2 H 6 O. Contact with the liquid can cause frostbite. Flash point near 190F.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Melting Point C Physical Form. The compound below is classified as a _____. The Williamson ether synthesis is an S N 2 reaction in which an alkoxide ion is a nucleophile that displaces a halide ion from an alkyl halide to give an ether. Solvent Synonyms Mol Wt BP C Linear Formula H-Signal Multi CDCl 3 D 2 O CD 3 OD CD 3 2 SO CD 3 2 CO CD 3 CN C 6 D 6 Acetic Acid Ethanoic acid 6005 118 CH 3COOH CH 3 s 213 208 199 195 196 196 155 Formic Acid Methanoic Acid 4602 101 HCOOH H s 802 822 808 818 811 803 1-Butanol n-Butanol 1-Hydroxybutane n-Butyl alcohol. Diethylene glycol monoethyl ether.

Analytical 63 ACS reagent 44 Puriss 35 Cell Culture 21 Reagent 18 Purum 17 Anhydrous 13 BioXtra 13 ReagentPlus 11 Plant 7 Show More. It is easily ignited. Dimethoxyethane is miscible with water. The List of Toxic Substances in Schedule 1 of the Canadian Environmental Protection Act 1999 CEPA 1999 includes substances that are considered to be toxic as defined in Section 64 of the Act. A dimethyl ether b methoxyethane c methylethyloxide d propyl ether e none of the above 21.
 Source: chegg.com
Source: chegg.com
Any leak can be either liquid or vapor. Its vapors are heavier than air. It is shipped as a liquefied gas under its vapor pressure. The List of Toxic Substances in Schedule 1 of the Canadian Environmental Protection Act 1999 CEPA 1999 includes substances that are considered to be toxic as defined in Section 64 of the Act. Diethylene glycol monoethyl ether appears as a colorless slightly viscous liquid with a mild pleasant odor.
 Source: study.com
Source: study.com
Solvent Synonyms Mol Wt BP C Linear Formula H-Signal Multi CDCl 3 D 2 O CD 3 OD CD 3 2 SO CD 3 2 CO CD 3 CN C 6 D 6 Acetic Acid Ethanoic acid 6005 118 CH 3COOH CH 3 s 213 208 199 195 196 196 155 Formic Acid Methanoic Acid 4602 101 HCOOH H s 802 822 808 818 811 803 1-Butanol n-Butanol 1-Hydroxybutane n-Butyl alcohol. Its vapors are heavier than air. Under prolonged exposure to fire or intense heat the containers may rupture violently and. Dimethyl ether is a colorless gas with a faint ethereal odor. The ether functional group does not have a characteristic IUPAC nomenclature suffix so it is necessary to designate it as a substituent.

Dimethyl ether DME also known as methoxymethane is the organic compound with the formula CH 3 OCH 3 simplified to C 2 H 6 O. It is easily ignited. Intramolecular Williamson ether synthesis occurs at rates that depend on the number of atoms in the. C 6 H 14 O 3 or CH 3 CH 2 OCH 2 CH 2 OCH 2 CH 2 OH. Diethylene glycol monoethyl ether.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is dimethyl ether formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.