What is lithium chloride formula
What Is Lithium Chloride Formula. NaOH HCl NaCl H 2 O. Copy this to my account. The names are found by finding the intersection between the cations and anions. Lithium bromide was used as a sedative beginning in the early 1900s but it fell into disfavor in the 1940s as newer sedatives became available and when some heart patients died after using the salt substitute lithium chloride.
 How To Write The Formula For Lithium Chloride Youtube From youtube.com
How To Write The Formula For Lithium Chloride Youtube From youtube.com
The names are found by finding the intersection between the cations and anions. Lithium chloride is a chemical compound with the formula Li ClThe salt is a typical ionic compound with certain covalent characters although the small size of the Li ion gives rise to properties not seen for other alkali metal chlorides such as extraordinary solubility in polar solvents 8305 g100 mL of water at 20 C and its hygroscopic properties. Potassium chloride is characterized by a colourless crystalline appearance and an odourless smell. Ionic Compounds Naming and Formula Writing. Shivendra dhar dwivedi August 8 2020 at 1235 pm. E-mail to a friend.
NaOH HCl NaCl H 2 O.
Ionic Compound Naming and Formula Writing List 1. Arrhenius acid and base reacts to form salt and water. Here negatively charged hydroxide ion reacts with positively charged hydrogen ion to form water. In its solid form potassium chloride. Sodium chloride is represented by NaCl meaning that sodium and chlorine ratio in sodium chloride is 1 to 1. Identifying an appropriate solid electrolyte is crucial for enabling the safe energy-dense all-solid-state Li batteries 123456Recently chloride superionic conductors were raised as an.

For example sodium hydroxide and hydrochloric acid reacts to form sodium chloride salt and water. Potassium chloride is an ionic salt featuring a bond between an alkali metal and a halogen. OH H H 2 O. What hydrate is it. The subscript numbers in an empirical formula.
 Source: youtube.com
Source: youtube.com
Dry Lithium chloride is the feed material for manufacture of Li metal by electrolysis. Like lithium carbonate and lithium chloride it was used as treatment for bipolar disorder. From now I have all formula in a single place. The mass of the hydrated salt obtained was 721 g. Thanks byjus my aspirations always with you.
 Source: coolgyan.org
Source: coolgyan.org
OH H H 2 O. In its solid form potassium chloride. The subscript numbers in an empirical formula. Ionic Compound Naming and Formula Writing List 1. The names are found by finding the intersection between the cations and anions.
 Source: youtube.com
Source: youtube.com
E-mail to a friend. From now I have all formula in a single place. Identifying an appropriate solid electrolyte is crucial for enabling the safe energy-dense all-solid-state Li batteries 123456Recently chloride superionic conductors were raised as an. Copy this to my account. Like lithium carbonate and lithium chloride it was used as treatment for bipolar disorder.
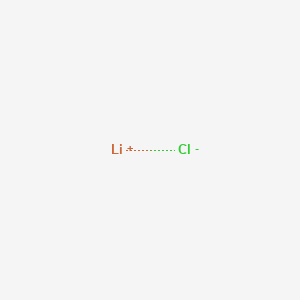
Since table salt is an ionic compound the formula implies that numbers of Na ions and Cl- ions are the same in the solid. Dry Lithium chloride is the feed material for manufacture of Li metal by electrolysis. E-mail to a friend. Copy this to my account. The subscript numbers in an empirical formula.
 Source: youtube.com
Source: youtube.com
From now I have all formula in a single place. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Binary Ionic Formula Practice Name_____ 1 sodium chloride Na1 Cl-1 NaCl 2 lithium bromide Li1 Br-1 LiBr 3 magnesium flouride Mg2 F-1 MgF2 4 potassium oxide K1 O-2 K2O 5 calcium sulfide Ca2 S-2 CaS 6 aluminum iodide Al3 I-1 AlI3 7 barium bromide Ba2 Br-1 BaBr2 8 aluminum sulfide Al3 S-2 Al2S 3 9 calcium phosphide P-3 Ca2 P-3. Here negatively charged hydroxide ion reacts with positively charged hydrogen ion to form water. Identifying an appropriate solid electrolyte is crucial for enabling the safe energy-dense all-solid-state Li batteries 123456Recently chloride superionic conductors were raised as an.
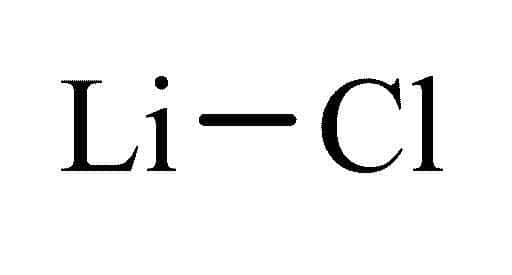 Source: philipharris.co.uk
Source: philipharris.co.uk
Here negatively charged hydroxide ion reacts with positively charged hydrogen ion to form water. Chemical Formula Writing Worksheet Two Write chemical formulas for the compounds in each box. Again the subscript 1 is omitted. Cations Anions zinc iron II iron III gallium silver lead IV chloride. The cell is operated at 400 to 420 C with a voltage across the cell of 8 to 9 volts.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Ionic Compound Naming and Formula Writing List 1. Dry Lithium chloride is the feed material for manufacture of Li metal by electrolysis. From now I have all formula in a single place. Thank for making this chart. Lithium bromide was used as a sedative beginning in the early 1900s but it fell into disfavor in the 1940s as newer sedatives became available and when some heart patients died after using the salt substitute lithium chloride.
 Source: youtube.com
Source: youtube.com
Ionic Compound Naming and Formula Writing List 1. Shivendra dhar dwivedi August 8 2020 at 1235 pm. Since table salt is an ionic compound the formula implies that numbers of Na ions and Cl- ions are the same in the solid. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown. OH H H 2 O.
 Source: coolgyan.org
Source: coolgyan.org
OH H H 2 O. LiClO 4— 478 g 10639 g. Utkarsh kt July 18 2020 at 225 pm. Like lithium carbonate and lithium chloride it was used as treatment for bipolar disorder. Anhydrous lithium perchlorate 478 g was dissolved in water and re-crystalized.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is lithium chloride formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.