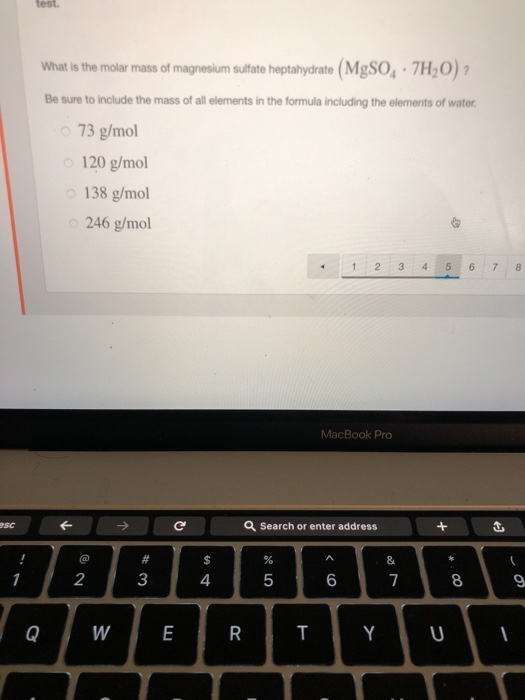
What is the molar mass of magnesium sulfate
What Is The Molar Mass Of Magnesium Sulfate. Accordingly 141 mg of elemental magnesium is contained in 1000 mg of magnesium glycinate. It is more crystalline when compared to starch. Molar Mass of Frequently Calculated Chemicals. Structure of Cellulose C 6 H 10 O 5n.
 Ppt The Mole Molar Mass And Molecular Formulas Powerpoint Presentation Id 9571373 From slideserve.com
Ppt The Mole Molar Mass And Molecular Formulas Powerpoint Presentation Id 9571373 From slideserve.com
It contains 141 elemental magnesium by mass. But starch goes from crystalline to. In high temperature It can be broken down into glucose by treating with concentrated minerals acids. The molar volume of a gas expresses the volume occupied by 1 mole of that respective gas under certain temperature and pressure conditions. Magnesium glycinate also known as magnesium diglycinate or magnesium bisglycinate is the magnesium salt of glycine one magnesium and two glycine molecules and is sold as a dietary supplement. Structure of Cellulose C 6 H 10 O 5n.
It contains 141 elemental magnesium by mass.
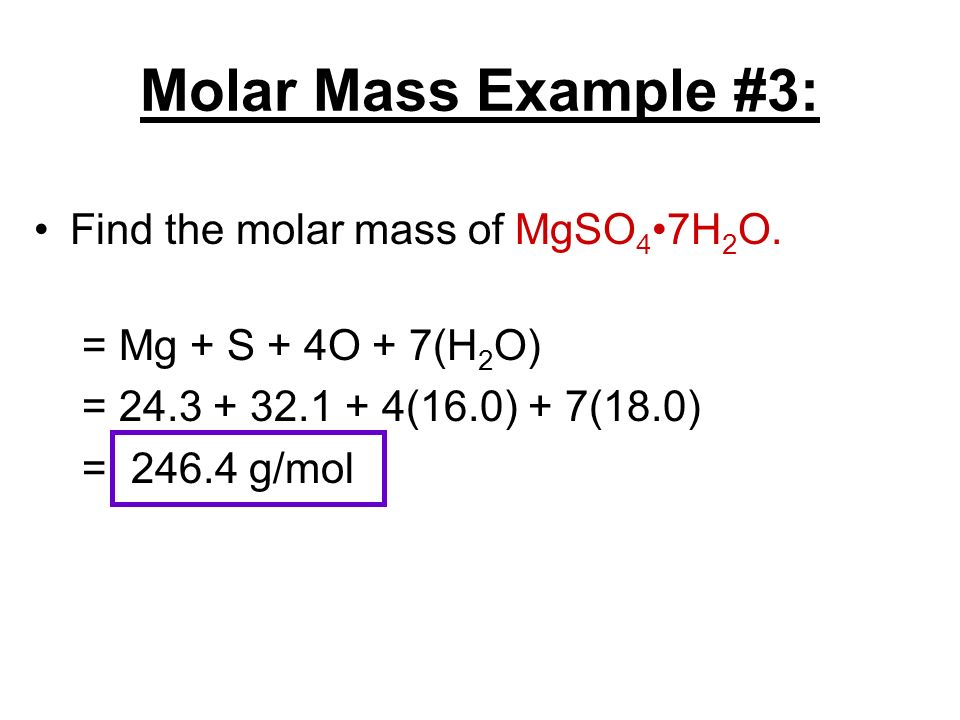
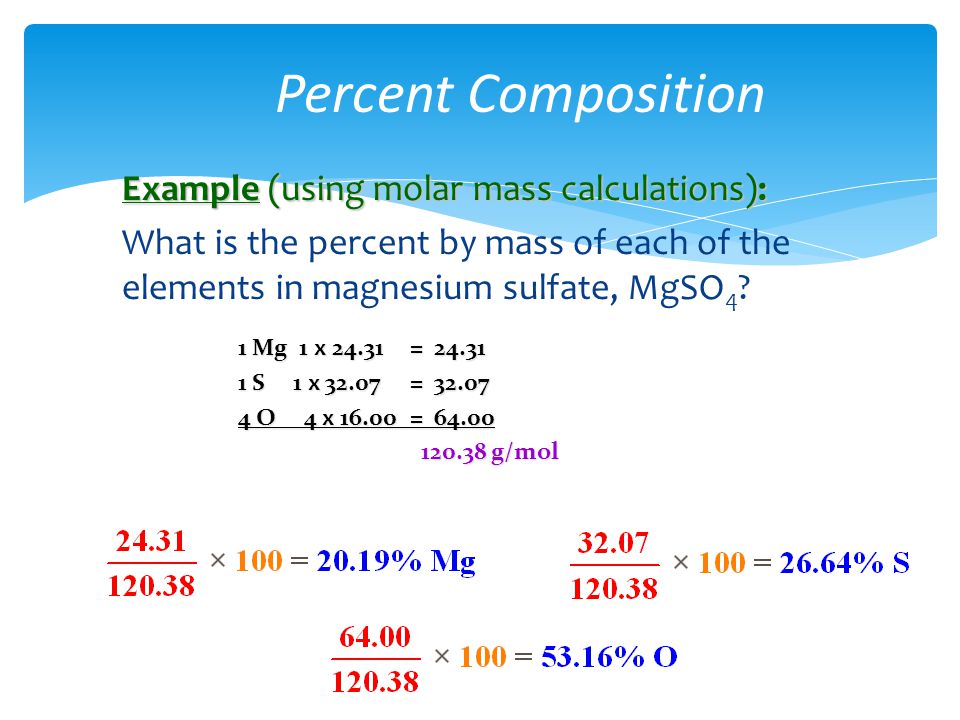
Structure of Cellulose C 6 H 10 O 5n. Calcium sulfate or calcium sulphate is the inorganic compound with the formula CaSO 4 and related hydratesIn the form of γ-anhydrite the anhydrous form it is used as a desiccantOne particular hydrate is better known as plaster of Paris and another occurs naturally as the mineral gypsumIt has many uses in industry. Calculate the molar mass of the following Magnesium nitrate MgNO 32 1 Mg 243050 2 N 2x 140067 280134 6 O 6 x 159994 959964 Molar mass of MgNO 32 1483148 g Calcium carbonate CaCO 3 1 Ca 40078 1 C 12011 3 O 3 x 159994 Molar mass of CaCO 3 100087 g IronII sulfate FeSO 4 Molar mass of FeSO 4 151909 g Chapter 3 Solutions Solution. All forms are white solids that are poorly soluble in water. Since you know that after complete dehydration the mass of the sample is equal to 482 g you can say that the hydrate contained 482 g - MgSO_4 Use the molar mass of the compound to convert this to moles 482 colorredcancelcolorblackg 1 mole MgSO_412037colorredcancelcolorblackg. Molar Mass of Frequently Calculated Chemicals.
 Source: slideplayer.com
Source: slideplayer.com
All forms are white solids that are poorly soluble in water. Calculate the molar mass of the following Magnesium nitrate MgNO 32 1 Mg 243050 2 N 2x 140067 280134 6 O 6 x 159994 959964 Molar mass of MgNO 32 1483148 g Calcium carbonate CaCO 3 1 Ca 40078 1 C 12011 3 O 3 x 159994 Molar mass of CaCO 3 100087 g IronII sulfate FeSO 4 Molar mass of FeSO 4 151909 g Chapter 3 Solutions Solution. Molar Mass of Frequently Calculated Chemicals. Magnesium glycinate also known as magnesium diglycinate or magnesium bisglycinate is the magnesium salt of glycine one magnesium and two glycine molecules and is sold as a dietary supplement. The most common example is the molar volume of a gas at STP Standard Temperature and Pressure which is equal to 224 L for 1 mole of any ideal gas at a temperature equal to 27315 K and a pressure equal to 100 atm.
 Source: chegg.com
Source: chegg.com
C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Structure of Cellulose C6H10O5n. Molecular Weight Molar Mass. The most common example is the molar volume of a gas at STP Standard Temperature and Pressure which is equal to 224 L for 1 mole of any ideal gas at a temperature equal to 27315 K and a pressure equal to 100 atm. All forms are white solids that are poorly soluble in water.

Calculate the molar mass of the following Magnesium nitrate MgNO 32 1 Mg 243050 2 N 2x 140067 280134 6 O 6 x 159994 959964 Molar mass of MgNO 32 1483148 g Calcium carbonate CaCO 3 1 Ca 40078 1 C 12011 3 O 3 x 159994 Molar mass of CaCO 3 100087 g IronII sulfate FeSO 4 Molar mass of FeSO 4 151909 g Chapter 3 Solutions Solution. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. But starch goes from crystalline to. Accordingly 141 mg of elemental magnesium is contained in 1000 mg of magnesium glycinate. Molecular Weight Molar Mass.
 Source: slideplayer.com
Source: slideplayer.com
It contains 141 elemental magnesium by mass. Molecular Weight Molar Mass. It is more crystalline when compared to starch. Structure of Cellulose C6H10O5n. But starch goes from crystalline to.
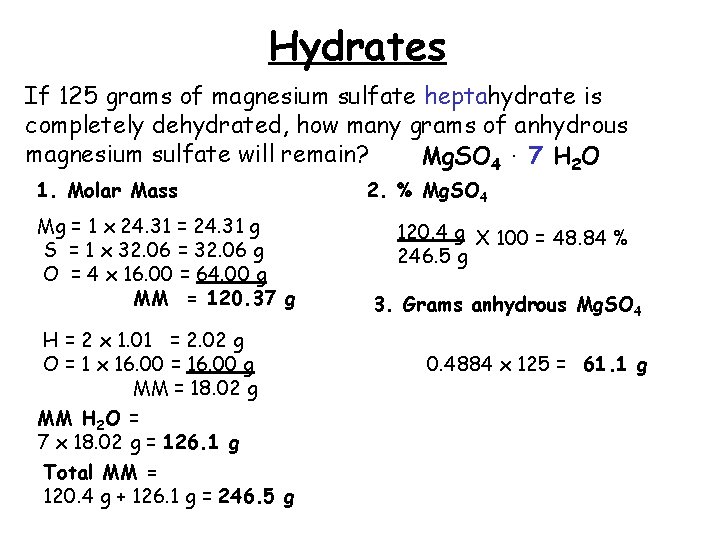 Source: slidetodoc.com
Source: slidetodoc.com
Since you know that after complete dehydration the mass of the sample is equal to 482 g you can say that the hydrate contained 482 g - MgSO_4 Use the molar mass of the compound to convert this to moles 482 colorredcancelcolorblackg 1 mole MgSO_412037colorredcancelcolorblackg. Accordingly 141 mg of elemental magnesium is contained in 1000 mg of magnesium glycinate. Calcium sulfate or calcium sulphate is the inorganic compound with the formula CaSO 4 and related hydratesIn the form of γ-anhydrite the anhydrous form it is used as a desiccantOne particular hydrate is better known as plaster of Paris and another occurs naturally as the mineral gypsumIt has many uses in industry. Structure of Cellulose C 6 H 10 O 5n. Molecular Weight Molar Mass.
 Source: slideserve.com
Source: slideserve.com
The molar volume of a gas expresses the volume occupied by 1 mole of that respective gas under certain temperature and pressure conditions. All forms are white solids that are poorly soluble in water. In your case the anhydrous salt is magnesium sulfate MgSO_4. Magnesium glycinate also known as magnesium diglycinate or magnesium bisglycinate is the magnesium salt of glycine one magnesium and two glycine molecules and is sold as a dietary supplement. Molar Mass of Frequently Calculated Chemicals.
 Source: youtube.com
Source: youtube.com
But starch goes from crystalline to. Molar Mass of Frequently Calculated Chemicals. Magnesium glycinate also known as magnesium diglycinate or magnesium bisglycinate is the magnesium salt of glycine one magnesium and two glycine molecules and is sold as a dietary supplement. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. In your case the anhydrous salt is magnesium sulfate MgSO_4.
 Source: slideplayer.com
Source: slideplayer.com
Molar Mass of Frequently Calculated Chemicals. Accordingly 141 mg of elemental magnesium is contained in 1000 mg of magnesium glycinate. Since you know that after complete dehydration the mass of the sample is equal to 482 g you can say that the hydrate contained 482 g - MgSO_4 Use the molar mass of the compound to convert this to moles 482 colorredcancelcolorblackg 1 mole MgSO_412037colorredcancelcolorblackg. But starch goes from crystalline to. Structure of Cellulose C6H10O5n.
 Source: youtube.com
Source: youtube.com
Molar Mass of Frequently Calculated Chemicals. Molecular Weight Molar Mass. But starch goes from crystalline to. It is more crystalline when compared to starch. Calculate the molar mass of the following Magnesium nitrate MgNO 32 1 Mg 243050 2 N 2x 140067 280134 6 O 6 x 159994 959964 Molar mass of MgNO 32 1483148 g Calcium carbonate CaCO 3 1 Ca 40078 1 C 12011 3 O 3 x 159994 Molar mass of CaCO 3 100087 g IronII sulfate FeSO 4 Molar mass of FeSO 4 151909 g Chapter 3 Solutions Solution.
 Source: youtube.com
Source: youtube.com
All forms are white solids that are poorly soluble in water. It contains 141 elemental magnesium by mass. Structure of Cellulose C 6 H 10 O 5n. Molar Mass of Frequently Calculated Chemicals. Calculate the molar mass of the following Magnesium nitrate MgNO 32 1 Mg 243050 2 N 2x 140067 280134 6 O 6 x 159994 959964 Molar mass of MgNO 32 1483148 g Calcium carbonate CaCO 3 1 Ca 40078 1 C 12011 3 O 3 x 159994 Molar mass of CaCO 3 100087 g IronII sulfate FeSO 4 Molar mass of FeSO 4 151909 g Chapter 3 Solutions Solution.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is the molar mass of magnesium sulfate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.