What is the molar mass of zinc sulfate
What Is The Molar Mass Of Zinc Sulfate. 136315 gmol Appearance white crystalline solid hygroscopic and very. So the molar mass is 6538 g mol-1. New Movies to Watch with Your Family this Thanksgiving. This comes to 0250 mol x 6538 g mol-1 163 g.
 Molar Mass Of Znso4 Brainly In From brainly.in
Molar Mass Of Znso4 Brainly In From brainly.in
Zinc chloride reacts with metal oxides MO to give derivatives of the. Take A Sneak Peak At The Movies Coming Out This Week 812 New Movie Releases This Weekend. The mass is calculated by moles x molar mass. This comes to 0250 mol x 6538 g mol-1 163 g. Illustrative is the preparation of zinc carbonate. What is the molarity of an aqueous solution of sodium hydroxide produced when 350 ml of a 540 M solution was diluted to 8900 ml.
351 g of hydrated zinc sulfate were heated and 197 g of anhydrous zinc sulfate were obtained.
New Movies to Watch with Your Family this Thanksgiving. Side effects of excess supplementation may include abdominal pain vomiting headache and tiredness. Zinc sulfate is an inorganic compoundIt is used as a dietary supplement to treat zinc deficiency and to prevent the condition in those at high risk. Formula triangle for moles mass and molar mass. From the periodic table the relative atomic mass of Zn is 6538. IDM Operations Laboratory Management Meetings for 2021 will be held via Microsoft Teams on the following Wednesdays.
 Source: slideplayer.com
Source: slideplayer.com
How many moles are in 264 g of sucrose C 12 H 11 O 22 M r 3423. IDM Operations Laboratory Management Meetings for 2021 will be held via Microsoft Teams on the following Wednesdays. How many grams of NaCl are required to prepare 985 mL of 077 M NaCl solution. From the periodic table the relative atomic mass of Zn is 6538. 136315 gmol Appearance white crystalline solid hygroscopic and very.
 Source: youtube.com
Source: youtube.com
And the sulfate can often be used interchangeably for the preparation of other zinc compounds. From the periodic table the relative atomic mass of Zn is 6538. So the molar mass is 6538 g mol-1. And the sulfate can often be used interchangeably for the preparation of other zinc compounds. The mass is calculated by moles x molar mass.
 Source: youtube.com
Source: youtube.com
And the sulfate can often be used interchangeably for the preparation of other zinc compounds. IDM Operations Laboratory Management Meetings for 2021 will be held via Microsoft Teams on the following Wednesdays. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. ZnCl 2 Na 2 CO 3 aq ZnCO 3 s 2 NaClaq Applications As a metallurgical flux. 136315 gmol Appearance white crystalline solid hygroscopic and very.
 Source: slideplayer.com
Source: slideplayer.com
A Google ingyenes szolgáltatása azonnal lefordítja a szavakat kifejezéseket és weboldalakat a magyar és több mint 100 további nyelv kombinációjában. IDM Operations Laboratory Management Meetings for 2021 will be held via Microsoft Teams on the following Wednesdays. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. How many moles are in 264 g of sucrose C 12 H 11 O 22 M r 3423. This comes to 0250 mol x 6538 g mol-1 163 g.
 Source: slideplayer.com
Source: slideplayer.com
Zinc chloride reacts with metal oxides MO to give derivatives of the. Zinc sulfate is an inorganic compoundIt is used as a dietary supplement to treat zinc deficiency and to prevent the condition in those at high risk. New Movies to Watch with Your Family this Thanksgiving. Illustrative is the preparation of zinc carbonate. Side effects of excess supplementation may include abdominal pain vomiting headache and tiredness.
 Source: brainly.in
Source: brainly.in
136315 gmol Appearance white crystalline solid hygroscopic and very. Molar Mass of Frequently Calculated Chemicals. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. The mass is calculated by moles x molar mass. What is the mass of 0250 moles of zinc.
 Source: slideserve.com
Source: slideserve.com
If the molar mass of the salt is 218 gmol what mass is required. Formula triangle for moles mass and molar mass. If the molar mass of the salt is 218 gmol what mass is required. How many grams of NaCl are required to prepare 985 mL of 077 M NaCl solution. A Google ingyenes szolgáltatása azonnal lefordítja a szavakat kifejezéseket és weboldalakat a magyar és több mint 100 további nyelv kombinációjában.
 Source: learnah.org
Source: learnah.org
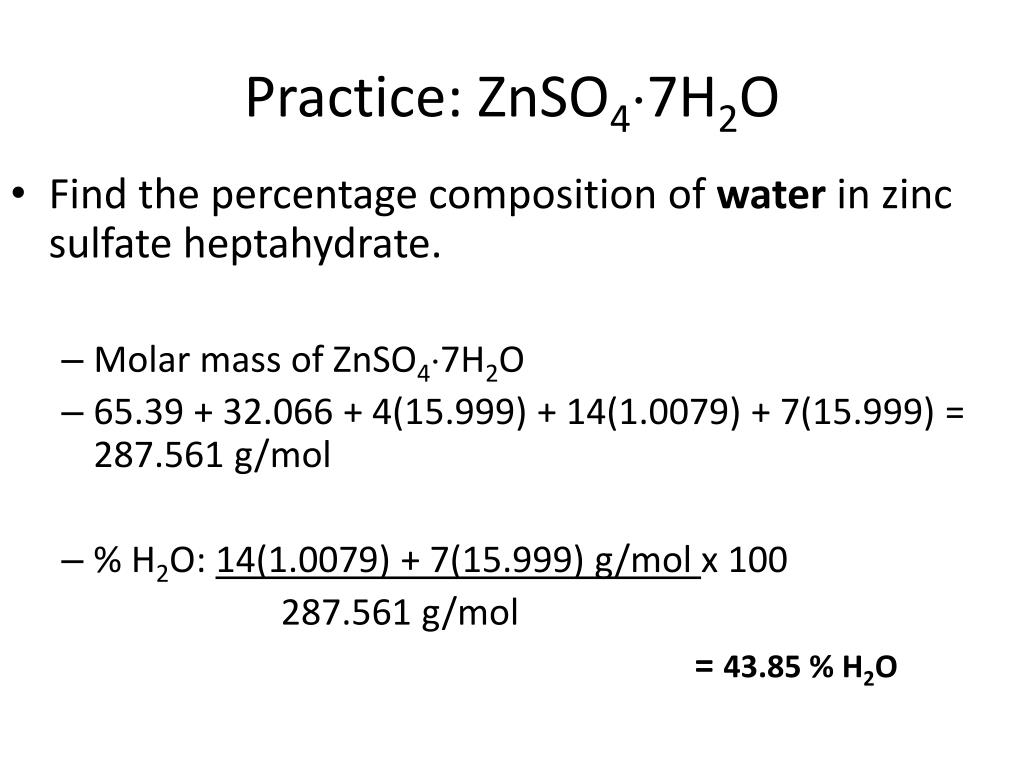
The most common form includes water of crystallization as the heptahydrate with the formula Zn SO 4 7H 2 O. The most common form includes water of crystallization as the heptahydrate with the formula Zn SO 4 7H 2 O. So the molar mass is 6538 g mol-1. A Google ingyenes szolgáltatása azonnal lefordítja a szavakat kifejezéseket és weboldalakat a magyar és több mint 100 további nyelv kombinációjában. Zinc chloride reacts with metal oxides MO to give derivatives of the.
 Source: brainly.in
Source: brainly.in
And the sulfate can often be used interchangeably for the preparation of other zinc compounds. Zinc chloride reacts with metal oxides MO to give derivatives of the. Formula triangle for moles mass and molar mass. How many grams of NaCl are required to prepare 985 mL of 077 M NaCl solution. If the molar mass of the salt is 218 gmol what mass is required.
 Source: toppr.com
Source: toppr.com
How many grams of NaCl are required to prepare 985 mL of 077 M NaCl solution. Take A Sneak Peak At The Movies Coming Out This Week 812 New Movie Releases This Weekend. If the molar mass of the salt is 218 gmol what mass is required. Calculate the value of the integer xin ZnSO4xH2O Calculate the mass of H2O 351 197 154g Calculate moles of ZnSO4 Calculate moles of H2O 197 1615 154 18 00122 0085 Calculate ratio of mole of ZnSO4 to H2O 00122 00122 0085 00122 1 7 X 7 N Goalby. What is the molarity of an aqueous solution of sodium hydroxide produced when 350 ml of a 540 M solution was diluted to 8900 ml.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the molar mass of zinc sulfate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.