What is the solubility of tin carbonate in water
What Is The Solubility Of Tin Carbonate In Water. There is a rapid disappearance alpha phase which is believed to be related to uptake by. SrSO 4 followed by strontianite strontium carbonate. If the pH is too low then the. Sodium carbonate Na 2 CO 3 is also adequately water soluble.
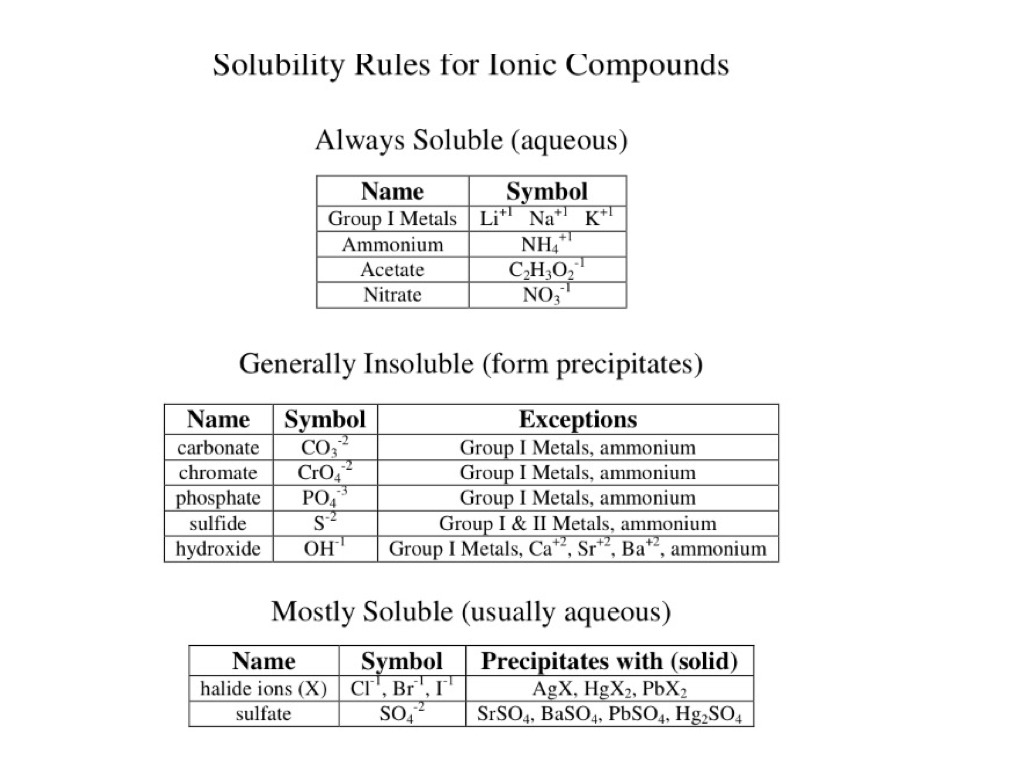
 Why Are Entries Missing On A Solubility Data Chart For Ionic Compounds Chemistry Stack Exchange From chemistry.stackexchange.com
Why Are Entries Missing On A Solubility Data Chart For Ionic Compounds Chemistry Stack Exchange From chemistry.stackexchange.com
Why is strontium present in water. At 20 o C solubility is 359 gL in other words adequately water soluble. By maintaining a relatively high pH around 10 the solubility of another lead compound that contributes to the protective layer leadII carbonate PbCO 3 decreases. Its rate of disappearance from the blood can be described by a two or possibly even three-compartment model. A solubility chart is a chart with a list of ions and how when mixed with other ions they can become precipitates or remain aqueous. Examples include strontium carbonate with a water solubility of 10 mgL and strontium chromate with a water solubility of 9 mgL.
Its rate of disappearance from the blood can be described by a two or possibly even three-compartment model.
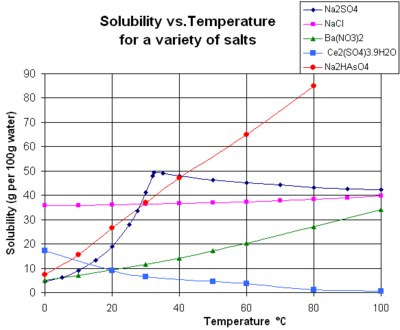
The following chart shows the solubility of multiple independent and various compounds in water at a pressure of 1 atm and at room temperature approx. There is a rapid disappearance alpha phase which is believed to be related to uptake by. A number of examples of water solubility of sodium are available. By maintaining a relatively high pH around 10 the solubility of another lead compound that contributes to the protective layer leadII carbonate PbCO 3 decreases. De most familiar sodium compounds is sodium chloride NaCl otherwise known as kitchen salt. All are paramagnetic green solids containing Ni 2.
Source:
The most significant strontium mineral is celestite strontium sulphate. Solubility is nearly temperature independent. The following chart shows the solubility of multiple independent and various compounds in water at a pressure of 1 atm and at room temperature approx. A number of examples of water solubility of sodium are available. At 20 o C solubility is 359 gL in other words adequately water soluble.

- Get the answer to this question and access a vast question bank that is tailored for students. 25 C 29815 K. SrSO 4 followed by strontianite strontium carbonate. The volume of distribution determined for lidocaine is 07 to 15 Lkg. - Get the answer to this question and access a vast question bank that is tailored for students.
 Source: porexfiltration.com
Source: porexfiltration.com
If the pH is too low then the. Strontium compounds can be water soluble. A number of examples of water solubility of sodium are available. The volume of distribution determined for lidocaine is 07 to 15 Lkg. - Get the answer to this question and access a vast question bank that is tailored for students.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Any box that reads soluble results in an aqueous product in which no precipitate has. At 20 o C solubility is 359 gL in other words adequately water soluble. The following chart shows the solubility of multiple independent and various compounds in water at a pressure of 1 atm and at room temperature approx. If the pH is too low then the. The volume of distribution determined for lidocaine is 07 to 15 Lkg.
 Source: youtube.com
Source: youtube.com
If the pH is too low then the. Second the pH of the water from the Flint water treatment plants was too low. By maintaining a relatively high pH around 10 the solubility of another lead compound that contributes to the protective layer leadII carbonate PbCO 3 decreases. 25 C 29815 K. At 20 o C solubility is 359 gL in other words adequately water soluble.
 Source: sciencedirect.com
Source: sciencedirect.com
- Get the answer to this question and access a vast question bank that is tailored for students. Classify the following into elements compounds and mixtures. Any box that reads soluble results in an aqueous product in which no precipitate has. 25 C 29815 K. The following chart shows the solubility of multiple independent and various compounds in water at a pressure of 1 atm and at room temperature approx.
Source: researchgate.net
54 Likes 13 Comments - UCLA VA Physiatry Residency uclava_pmrresidency on Instagram. Classify the following into elements compounds and mixtures. If the pH is too low then the. Strontium compounds can be water soluble. This helps to maintain the protective layer on the pipes.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
25 C 29815 K. Solubility is nearly temperature independent. 54 Likes 13 Comments - UCLA VA Physiatry Residency uclava_pmrresidency on Instagram. NickelII carbonate describes one or a mixture of inorganic compounds containing nickel and carbonateFrom the industrial perspective the most important nickel carbonate is basic nickel carbonate with the formula Ni 4 CO 3 OH 6 H 2 O 4Simpler carbonates ones more likely encountered in the laboratory are NiCO 3 and its hexahydrate. Any box that reads soluble results in an aqueous product in which no precipitate has.
 Source: socratic.org
Source: socratic.org
This helps to maintain the protective layer on the pipes. By maintaining a relatively high pH around 10 the solubility of another lead compound that contributes to the protective layer leadII carbonate PbCO 3 decreases. There is a rapid disappearance alpha phase which is believed to be related to uptake by. Its rate of disappearance from the blood can be described by a two or possibly even three-compartment model. A number of examples of water solubility of sodium are available.

NickelII carbonate describes one or a mixture of inorganic compounds containing nickel and carbonateFrom the industrial perspective the most important nickel carbonate is basic nickel carbonate with the formula Ni 4 CO 3 OH 6 H 2 O 4Simpler carbonates ones more likely encountered in the laboratory are NiCO 3 and its hexahydrate. A solubility chart is a chart with a list of ions and how when mixed with other ions they can become precipitates or remain aqueous. There is a rapid disappearance alpha phase which is believed to be related to uptake by. 25 C 29815 K. Classify the following into elements compounds and mixtures.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is the solubility of tin carbonate in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.