What the solubility of chromium hydroxide
What The Solubility Of Chromium Hydroxide. FeOH 3 s Fe 3 aq 3 OH-aq 1 The corresponding equilibrium expression is. For example the solubility of Fe 3 ions in basic solution is governed by the reaction. Use solubility rulesactivity tables and tables for strong bases and acids to write the equations. Calcium chromate solubility is 170 gL and at 0 o C calcium hypo chlorate solubility is 218 gL.
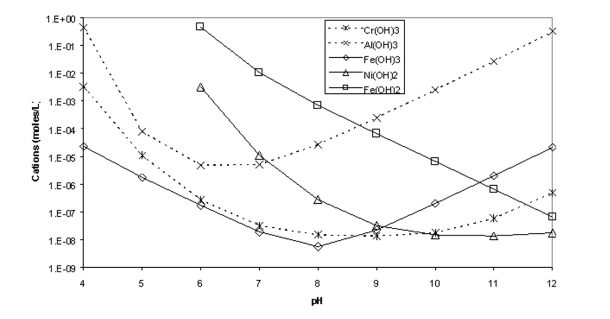 Solved The Graph Below Shows Solubility Vs Ph For Five Chegg Com From chegg.com
Solved The Graph Below Shows Solubility Vs Ph For Five Chegg Com From chegg.com
Equation 2 can be solved for the solubility. Solubility of iron and iron compounds. Elementary iron dissolves in water under normal conditions. Many iron compounds share this characteristic. Ksp Fe 3OH-3 2 The K sp of 63x10-38 shows that FeOH 3 is a very sparingly soluble salt under most conditions. ChromiumVI ChromiumVI compounds are oxidants at low or neutral pH.
58000 and Turkey Red is an organic compound with formula C 14 H 8 O 4 that has been used throughout history as a prominent red dye principally for dyeing textile fabricsHistorically it was derived from the roots of plants of the madder genus.
Calcium chromate solubility is 170 gL and at 0 o C calcium hypo chlorate solubility is 218 gL. Equation 2 can be solved for the solubility. ChromiumVI ChromiumVI compounds are oxidants at low or neutral pH. The water solubility of some iron compounds increases at lower pH values. ChromiumIII hydroxide CrOH 3 is amphoteric dissolving in acidic solutions to form CrH 2 O 6 3 and in basic solutions to form CrOH 6 3. Solubility of iron and iron compounds.
 Source: chegg.com
Source: chegg.com
Naturally occurring iron oxide iron hydroxide iron carbide and iron penta carbonyl are water insoluble. Many hydroxide salts are also quite insoluble. Alizarin also known as 12-dihydroxyanthraquinone Mordant Red 11 CI. Elementary iron dissolves in water under normal conditions. In 1869 it became the first natural dye to be produced synthetically.
 Source: researchgate.net
Source: researchgate.net
Solubility of other calcium compounds lies between the levels of these examples for example calcium arsenate 140 mgL calcium hydroxide 13 gL and calcium sulphate 27-88 gL. The nonabsorbable form of chromium 51Cr2O3 and water-soluble and more absorbable Na251CrO4 the hexavalent form of Cr were comparedTotal retention of chromium given orally ranged around 15 percent of the dose regardless. Calcium chromate solubility is 170 gL and at 0 o C calcium hypo chlorate solubility is 218 gL. Other iron compounds may be more water soluble than the examples. Calcium phosphate solubility is 20 mgL and that of calcium fluoride is 16 mgL.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Calcium phosphate solubility is 20 mgL and that of calcium fluoride is 16 mgL. Many hydroxide salts are also quite insoluble. Elementary iron dissolves in water under normal conditions. 1 Nickel IIIchloride potassium phosphate – Molecular equation. For example the solubility of Fe 3 ions in basic solution is governed by the reaction.
 Source: researchgate.net
Source: researchgate.net
Calcium phosphate solubility is 20 mgL and that of calcium fluoride is 16 mgL. ChromiumIII hydroxide CrOH 3 is amphoteric dissolving in acidic solutions to form CrH 2 O 6 3 and in basic solutions to form CrOH 6 3. Many iron compounds share this characteristic. Elementary iron dissolves in water under normal conditions. Alizarin also known as 12-dihydroxyanthraquinone Mordant Red 11 CI.
 Source: homeworklib.com
Source: homeworklib.com
Elementary iron dissolves in water under normal conditions. In 1869 it became the first natural dye to be produced synthetically. FeOH 3 s Fe 3 aq 3 OH-aq 1 The corresponding equilibrium expression is. Naturally occurring iron oxide iron hydroxide iron carbide and iron penta carbonyl are water insoluble. The acute and subacute toxicities of several CrIII and CrVI compounds chromium3 chloride chromium3 nitrate chromium3 sulfate chromium trioxide potassium dichromate were determined in NZC and CxO mice injected ipThe distal median lethal doses 10 days after treatment averaged 179 or - 18 X 10-6 g chromiumg body wt regardless of the oxidation state of the Cr.
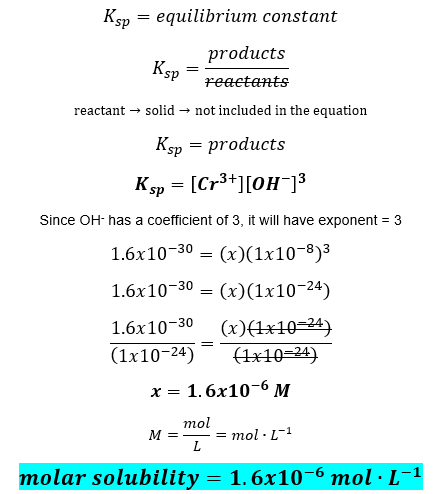 Source: clutchprep.com
Source: clutchprep.com
The nonabsorbable form of chromium 51Cr2O3 and water-soluble and more absorbable Na251CrO4 the hexavalent form of Cr were comparedTotal retention of chromium given orally ranged around 15 percent of the dose regardless. Use solubility rulesactivity tables and tables for strong bases and acids to write the equations. Calcium chromate solubility is 170 gL and at 0 o C calcium hypo chlorate solubility is 218 gL. Many iron compounds share this characteristic. Many hydroxide salts are also quite insoluble.
 Source: porexfiltration.com
Source: porexfiltration.com
The acute and subacute toxicities of several CrIII and CrVI compounds chromium3 chloride chromium3 nitrate chromium3 sulfate chromium trioxide potassium dichromate were determined in NZC and CxO mice injected ipThe distal median lethal doses 10 days after treatment averaged 179 or - 18 X 10-6 g chromiumg body wt regardless of the oxidation state of the Cr. Calcium phosphate solubility is 20 mgL and that of calcium fluoride is 16 mgL. Many iron compounds share this characteristic. Solubility of other calcium compounds lies between the levels of these examples for example calcium arsenate 140 mgL calcium hydroxide 13 gL and calcium sulphate 27-88 gL. In 1869 it became the first natural dye to be produced synthetically.
 Source: clutchprep.com
Source: clutchprep.com
It is dehydrated by heating to form the green chromiumIII oxide Cr 2 O 3 a stable oxide with a crystal structure identical to that of corundum. 1 Nickel IIIchloride potassium phosphate – Molecular equation. Equation 2 can be solved for the solubility. The intestinal absorption of trivalent and hexavalent chromium Cr given orally experiment I or infused in the intestine experiment II was investigated in rats. Solubility of other calcium compounds lies between the levels of these examples for example calcium arsenate 140 mgL calcium hydroxide 13 gL and calcium sulphate 27-88 gL.
 Source: chegg.com
Source: chegg.com
ChromiumIII hydroxide CrOH 3 is amphoteric dissolving in acidic solutions to form CrH 2 O 6 3 and in basic solutions to form CrOH 6 3. For example the solubility of Fe 3 ions in basic solution is governed by the reaction. Many iron compounds share this characteristic. Naturally occurring iron oxide iron hydroxide iron carbide and iron penta carbonyl are water insoluble. 1 Nickel IIIchloride potassium phosphate – Molecular equation.
 Source: youtube.com
Source: youtube.com
Solubility of other calcium compounds lies between the levels of these examples for example calcium arsenate 140 mgL calcium hydroxide 13 gL and calcium sulphate 27-88 gL. FeOH 3 s Fe 3 aq 3 OH-aq 1 The corresponding equilibrium expression is. The intestinal absorption of trivalent and hexavalent chromium Cr given orally experiment I or infused in the intestine experiment II was investigated in rats. Solubility of other calcium compounds lies between the levels of these examples for example calcium arsenate 140 mgL calcium hydroxide 13 gL and calcium sulphate 27-88 gL. Many iron compounds share this characteristic.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what the solubility of chromium hydroxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






