Which is stronger sodium chlorite or chloramines
Which Is Stronger Sodium Chlorite Or Chloramines. Mc Graw Hill-Water and Wastewater Engineering 2010 RETAi L EBook-Di Gi Book. It is a very. Chlorine dioxide has the advantage that it produces less harmful byproducts than chlorine. Chlorine dioxide gas is used to sterilize medical and laboratory equipment surfaces rooms and tools.
 Chloramines As A Disinfectant From lenntech.com
Chloramines As A Disinfectant From lenntech.com
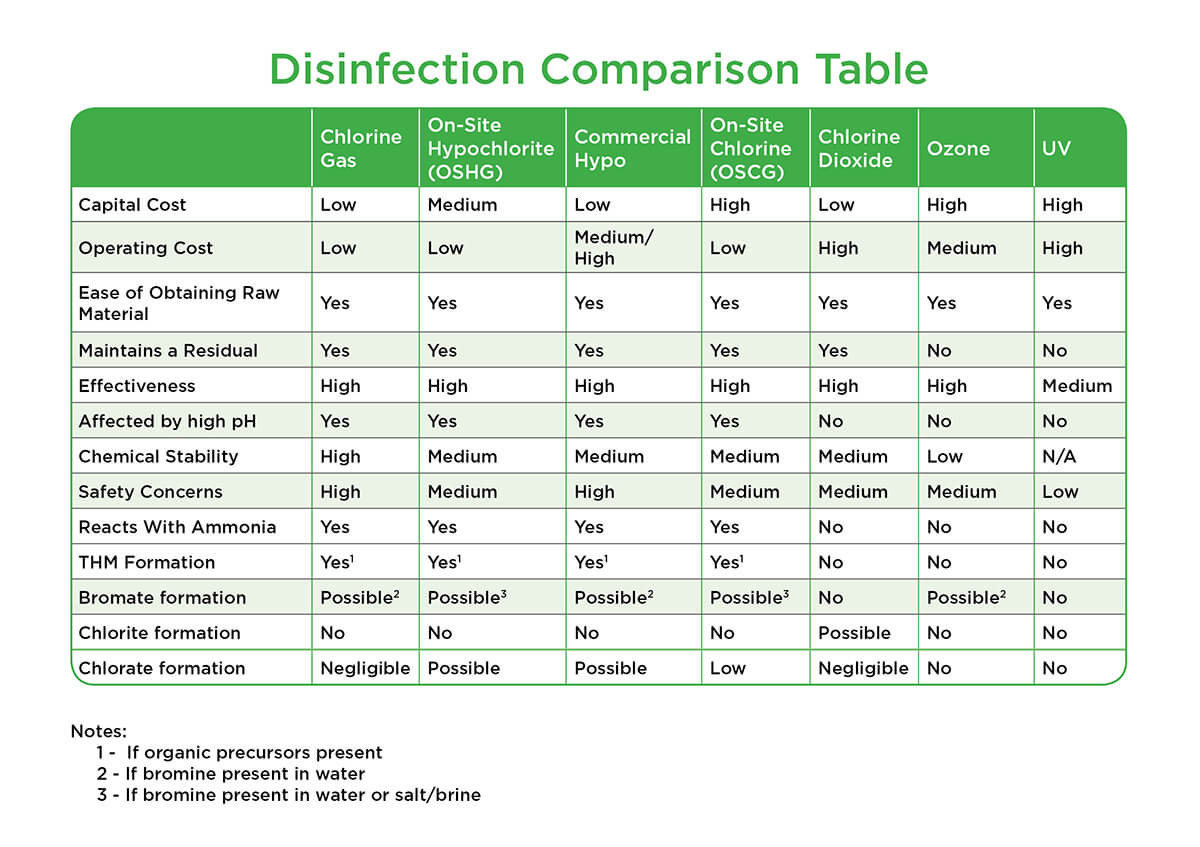
In solution it is a dissolved gas which makes it largely unaffected by pH but volatile and relatively easily stripped from solution. Chlorine Dose Required for NH3 - C12 Reaction 6-3 Table 6-2. Chlorine dioxide gas is used to sterilize medical and laboratory equipment surfaces rooms and tools. Chlorine dioxide has the advantage that it produces less harmful byproducts than chlorine. It is a very. It produces a clearer and stronger fiber than chlorine does.
Time to 99 Percent Conversion of Chlorine to Monochloramine 6-4 Table 6.
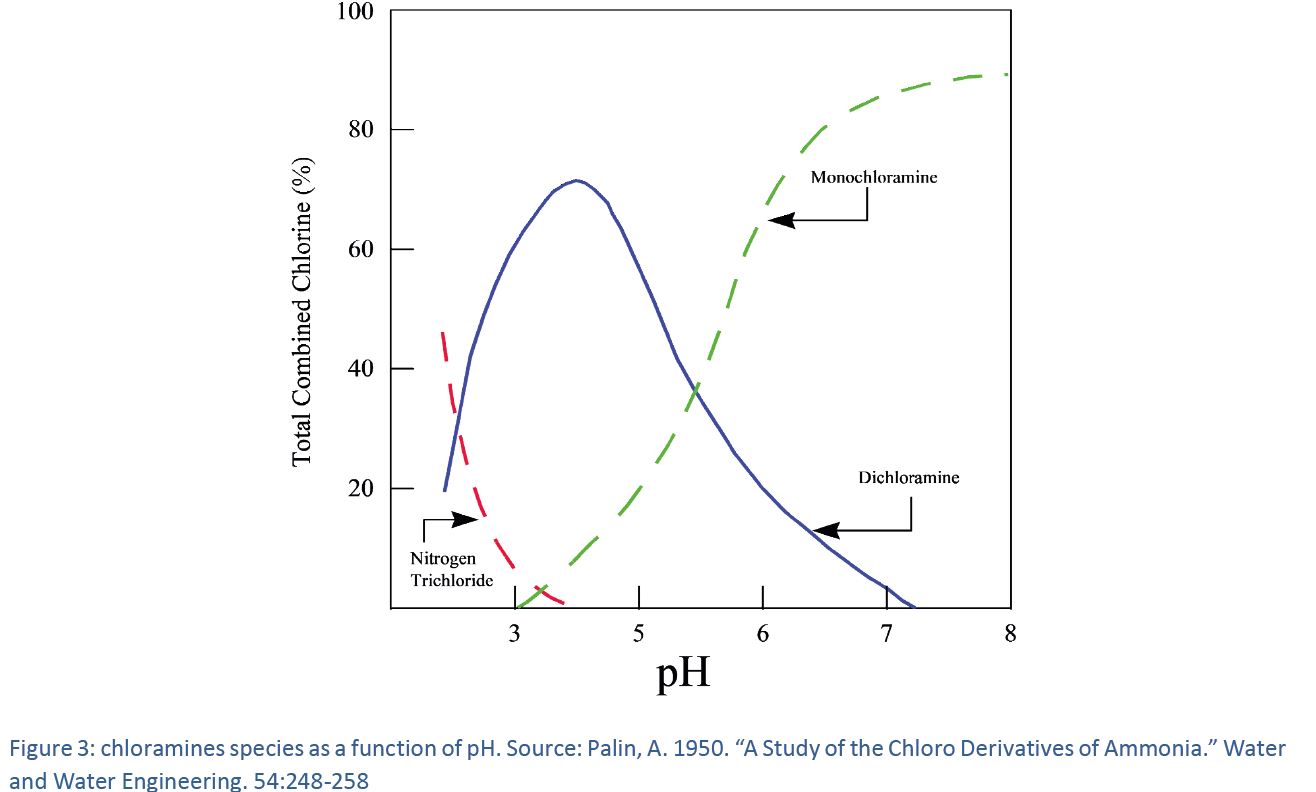
Typically pH values in the range of 35 to 55 are preferred as more acidic conditions ie pH 25 to 3 lead to the formation of chlorate Gates et al 2009. Chloric acid HOClO 2 is a strong acid that is quite stable in cold water up to 30 concentration but on warming. For potable water applications chlorine dioxide is generated on-site from sodium chlorite in reaction with either gaseous chlorine or hypochlorous acid under acidic conditions. However sodium chlorite is a stable salt and is useful for bleaching and stripping textiles as an oxidising agent and as a source of chlorine dioxide. Properties of Sodium Chlorite as Commercially Available 4-32 Table 4-5. This can be seen from the.
 Source: lenntech.com
Source: lenntech.com
This can be seen from the. Chlorine Dose Required for NH3 - C12 Reaction 6-3 Table 6-2. It is a very. This can be seen from the. Standard Methods For the Examination of Water and Wastewater 23nd edition.
 Source: researchgate.net
Source: researchgate.net
Chlorine Dose Required for NH3 - C12 Reaction 6-3 Table 6-2. Properties of Sodium Chlorite as Commercially Available 4-32 Table 4-5. Summary of Potassium Permanganate Use 5-12 Table 6-1. Chlorine is a weaker oxidising agent than fluorine but a stronger one than bromine or iodine. Chlorine Dose Required for NH3 - C12 Reaction 6-3 Table 6-2.
 Source: sanipur.com
Source: sanipur.com
Typically pH values in the range of 35 to 55 are preferred as more acidic conditions ie pH 25 to 3 lead to the formation of chlorate Gates et al 2009. Properties of Sodium Chlorite as Commercially Available 4-32 Table 4-5. Chlorine dioxide characteristics are quite different from chlorine. For potable water applications chlorine dioxide is generated on-site from sodium chlorite in reaction with either gaseous chlorine or hypochlorous acid under acidic conditions. Chlorine dioxide is also a strong disinfectant and a.
 Source: denora.com
Source: denora.com
Summary of Potassium Permanganate Use 5-12 Table 6-1. Mc Graw Hill-Water and Wastewater Engineering 2010 RETAi L EBook-Di Gi Book. Chloric acid HOClO 2 is a strong acid that is quite stable in cold water up to 30 concentration but on warming. Potassium Permanganate CT Values for 2-log Inactivation of MS-2 Bacteriophage 5-7 Table 5-2. It produces a clearer and stronger fiber than chlorine does.
 Source: sciencedirect.com
Source: sciencedirect.com
Potassium Permanganate CT Values for 2-log Inactivation of MS-2 Bacteriophage 5-7 Table 5-2. Properties of Sodium Chlorite as Commercially Available 4-32 Table 4-5. For large installations sodium chlorite chlorine gas Cl 2. Summary for Chlorine Dioxide 4-34 Table 5-1. In solution it is a dissolved gas which makes it largely unaffected by pH but volatile and relatively easily stripped from solution.
 Source: infinitabiotech.com
Source: infinitabiotech.com
In solution it is a dissolved gas which makes it largely unaffected by pH but volatile and relatively easily stripped from solution. Mc Graw Hill-Water and Wastewater Engineering 2010 RETAi L EBook-Di Gi Book. Properties of Sodium Chlorite as Commercially Available 4-32 Table 4-5. For large installations sodium chlorite chlorine gas Cl 2. Chlorine dioxide characteristics are quite different from chlorine.
 Source: sciencedirect.com
Source: sciencedirect.com
However sodium chlorite is a stable salt and is useful for bleaching and stripping textiles as an oxidising agent and as a source of chlorine dioxide. Typically pH values in the range of 35 to 55 are preferred as more acidic conditions ie pH 25 to 3 lead to the formation of chlorate Gates et al 2009. Time to 99 Percent Conversion of Chlorine to Monochloramine 6-4 Table 6. Chlorine Dose Required for NH3 - C12 Reaction 6-3 Table 6-2. Standard Methods For the Examination of Water and Wastewater 23nd edition.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Chloric acid HOClO 2 is a strong acid that is quite stable in cold water up to 30 concentration but on warming. Standard Methods For the Examination of Water and Wastewater 23nd edition. Summary of Potassium Permanganate Use 5-12 Table 6-1. It produces a clearer and stronger fiber than chlorine does. For large installations sodium chlorite chlorine gas Cl 2.
 Source: blog.chloramineconsulting.com
Source: blog.chloramineconsulting.com
Chlorine Dose Required for NH3 - C12 Reaction 6-3 Table 6-2. Chlorine dioxide has the advantage that it produces less harmful byproducts than chlorine. It is a very. However sodium chlorite is a stable salt and is useful for bleaching and stripping textiles as an oxidising agent and as a source of chlorine dioxide. Chlorine dioxide characteristics are quite different from chlorine.
Source: quora.com
It is a very. Potassium Permanganate CT Values for 2-log Inactivation of MS-2 Bacteriophage 5-7 Table 5-2. Chlorine is a weaker oxidising agent than fluorine but a stronger one than bromine or iodine. Chlorine Dose Required for NH3 - C12 Reaction 6-3 Table 6-2. Chlorine dioxide has the advantage that it produces less harmful byproducts than chlorine.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title which is stronger sodium chlorite or chloramines by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.