Will ethanol dissolve in carbon tetrachloride
Will Ethanol Dissolve In Carbon Tetrachloride. The vapor pressure of pure carbon tetrachloride is 9132 mmHg and its density is 15940 gcm3. If this solution is ideal its total vapor pressure at 20C is. PºCCl4 at 65ºC 531 torr Molar mass of CCl4 is 15381 gmol Molar mass of I2 is 2538 gmol. The vapor pressure of pure benzene at this temperature is 7461 mmHg and its density is 087865 gcm3.
 Solved Predict Whether The Following Solutes Will Be More Chegg Com From chegg.com
Solved Predict Whether The Following Solutes Will Be More Chegg Com From chegg.com
If this solution is ideal its total vapor pressure at 20C is. For the following reaction 309 grams of methane are allowed to react with 338 grams of carbon tetrachloride. What is the maximum amount of dichloromethane that. A solution is 4000 by volume benzene C6H6 in carbon tetrachloride at 20C. Dissolve 1361 g ofsodium acetate R in 500 mL of water R. Shake twice with a freshly prepared filtered 01 gL solution of dithizone R in chloroform RShakewithcarbon tetrachloride R until the extract is colourless.
The vapor pressure of pure carbon tetrachloride is 9132 mmHg and its density is 15940 gcm3.
Mix 250 mL of this solution with 250 mL of dilute acetic acid R. The vapor pressure of pure benzene at this temperature is 7461 mmHg and its density is 087865 gcm3. What is the maximum amount of dichloromethane that. If this solution is ideal its total vapor pressure at 20C is. PºCCl4 at 65ºC 531 torr Molar mass of CCl4 is 15381 gmol Molar mass of I2 is 2538 gmol. Shake twice with a freshly prepared filtered 01 gL solution of dithizone R in chloroform RShakewithcarbon tetrachloride R until the extract is colourless.
 Source: clutchprep.com
Source: clutchprep.com
PºCCl4 at 65ºC 531 torr Molar mass of CCl4 is 15381 gmol Molar mass of I2 is 2538 gmol. For the following reaction 309 grams of methane are allowed to react with 338 grams of carbon tetrachloride. Filter the aqueous layer to remove traces of carbon tetrachloride. The vapor pressure of pure carbon tetrachloride is 9132 mmHg and its density is 15940 gcm3. The vapor pressure of pure benzene at this temperature is 7461 mmHg and its density is 087865 gcm3.
 Source: slideplayer.com
Source: slideplayer.com
What is the maximum amount of dichloromethane that. PºCCl4 at 65ºC 531 torr Molar mass of CCl4 is 15381 gmol Molar mass of I2 is 2538 gmol. What is the maximum amount of dichloromethane that. Dissolve 1361 g ofsodium acetate R in 500 mL of water R. The vapor pressure of pure carbon tetrachloride is 9132 mmHg and its density is 15940 gcm3.
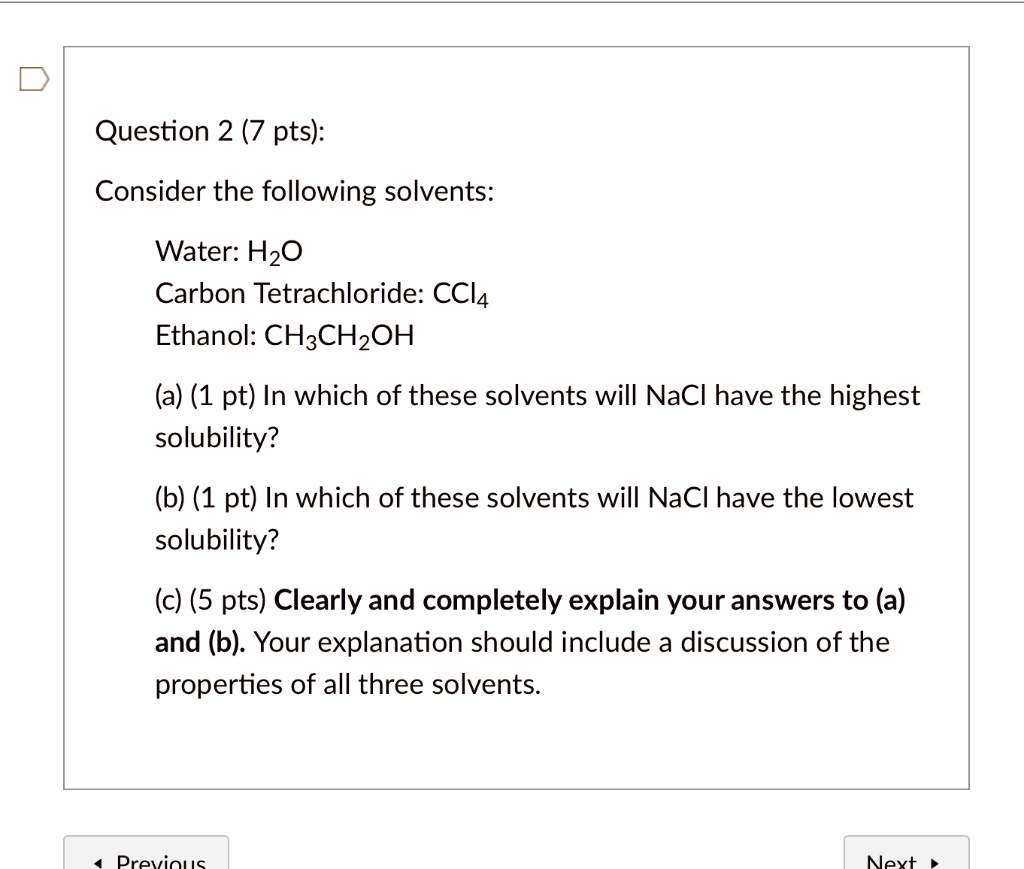 Source: numerade.com
Source: numerade.com
Shake twice with a freshly prepared filtered 01 gL solution of dithizone R in chloroform RShakewithcarbon tetrachloride R until the extract is colourless. The vapor pressure of pure carbon tetrachloride is 9132 mmHg and its density is 15940 gcm3. For the following reaction 309 grams of methane are allowed to react with 338 grams of carbon tetrachloride. The vapor pressure of pure benzene at this temperature is 7461 mmHg and its density is 087865 gcm3. PºCCl4 at 65ºC 531 torr Molar mass of CCl4 is 15381 gmol Molar mass of I2 is 2538 gmol.
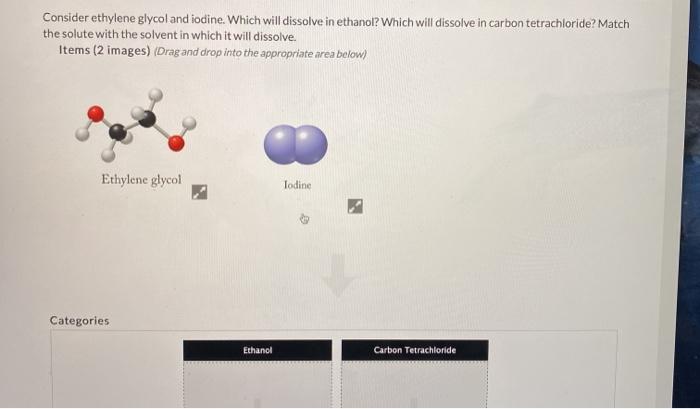
Dissolve 1361 g ofsodium acetate R in 500 mL of water R. What is the maximum amount of dichloromethane that. The vapor pressure of pure benzene at this temperature is 7461 mmHg and its density is 087865 gcm3. The vapor pressure of pure carbon tetrachloride is 9132 mmHg and its density is 15940 gcm3. Dissolve 1361 g ofsodium acetate R in 500 mL of water R.
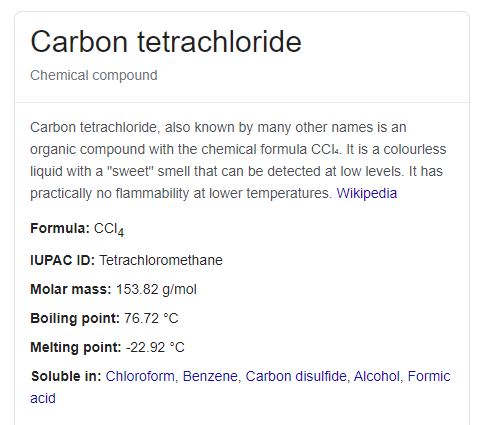 Source: waterfilteradvisor.com
Source: waterfilteradvisor.com
A solution is 4000 by volume benzene C6H6 in carbon tetrachloride at 20C. The vapor pressure of pure benzene at this temperature is 7461 mmHg and its density is 087865 gcm3. Shake twice with a freshly prepared filtered 01 gL solution of dithizone R in chloroform RShakewithcarbon tetrachloride R until the extract is colourless. PºCCl4 at 65ºC 531 torr Molar mass of CCl4 is 15381 gmol Molar mass of I2 is 2538 gmol. For the following reaction 309 grams of methane are allowed to react with 338 grams of carbon tetrachloride.
 Source: chegg.com
Source: chegg.com
Dissolve 1361 g ofsodium acetate R in 500 mL of water R. PºCCl4 at 65ºC 531 torr Molar mass of CCl4 is 15381 gmol Molar mass of I2 is 2538 gmol. The vapor pressure of pure carbon tetrachloride is 9132 mmHg and its density is 15940 gcm3. Predict the vapor pressure in torr of carbon tetrachloride CCl4 over a solution which is composed of 3750 g of CCl4 and 8840 g of I2 at 65ºC. The vapor pressure of pure benzene at this temperature is 7461 mmHg and its density is 087865 gcm3.
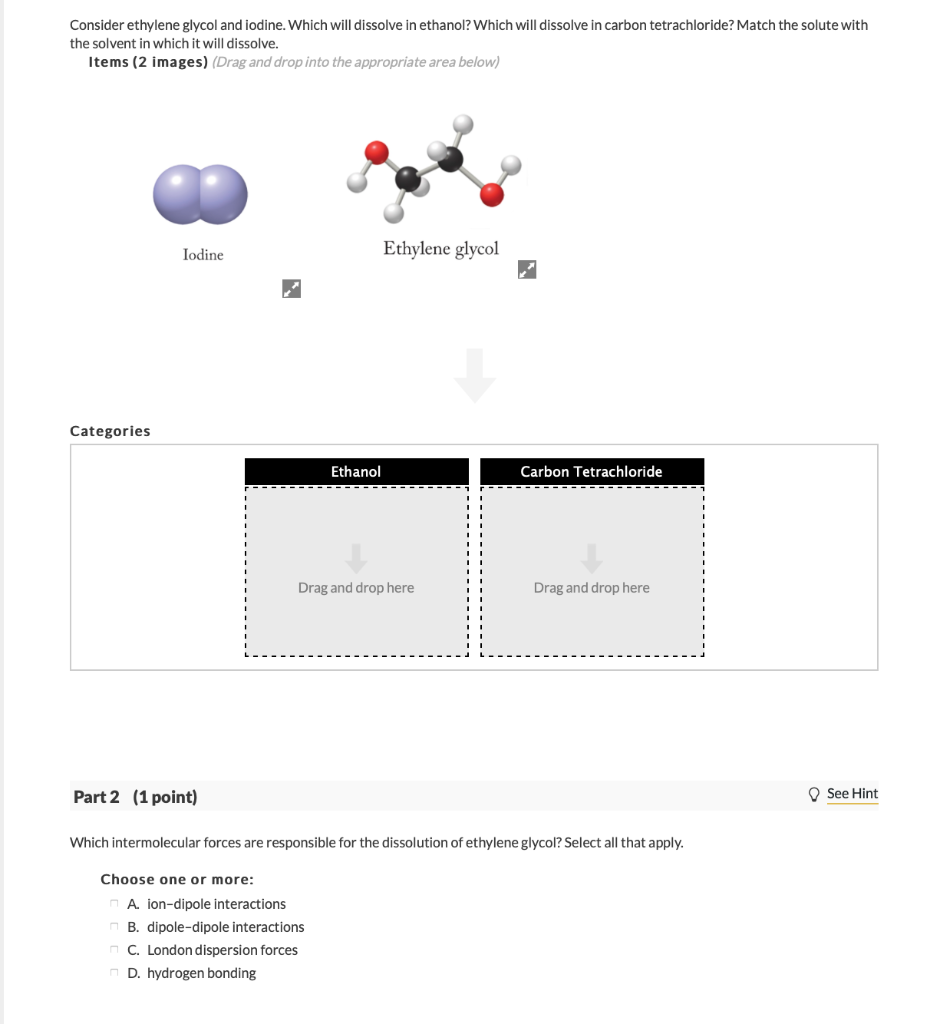 Source: chegg.com
Source: chegg.com
The vapor pressure of pure carbon tetrachloride is 9132 mmHg and its density is 15940 gcm3. PºCCl4 at 65ºC 531 torr Molar mass of CCl4 is 15381 gmol Molar mass of I2 is 2538 gmol. If this solution is ideal its total vapor pressure at 20C is. The vapor pressure of pure carbon tetrachloride is 9132 mmHg and its density is 15940 gcm3. Dissolve 1361 g ofsodium acetate R in 500 mL of water R.
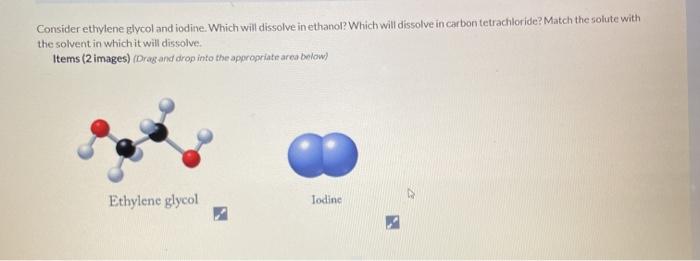
A solution is 4000 by volume benzene C6H6 in carbon tetrachloride at 20C. PºCCl4 at 65ºC 531 torr Molar mass of CCl4 is 15381 gmol Molar mass of I2 is 2538 gmol. Predict the vapor pressure in torr of carbon tetrachloride CCl4 over a solution which is composed of 3750 g of CCl4 and 8840 g of I2 at 65ºC. Dissolve 1361 g ofsodium acetate R in 500 mL of water R. What is the maximum amount of dichloromethane that.
 Source: chegg.com
Source: chegg.com
Filter the aqueous layer to remove traces of carbon tetrachloride. PºCCl4 at 65ºC 531 torr Molar mass of CCl4 is 15381 gmol Molar mass of I2 is 2538 gmol. The vapor pressure of pure benzene at this temperature is 7461 mmHg and its density is 087865 gcm3. Mix 250 mL of this solution with 250 mL of dilute acetic acid R. Dissolve 1361 g ofsodium acetate R in 500 mL of water R.
 Source: issuu.com
Source: issuu.com
A solution is 4000 by volume benzene C6H6 in carbon tetrachloride at 20C. PºCCl4 at 65ºC 531 torr Molar mass of CCl4 is 15381 gmol Molar mass of I2 is 2538 gmol. If this solution is ideal its total vapor pressure at 20C is. A solution is 4000 by volume benzene C6H6 in carbon tetrachloride at 20C. The vapor pressure of pure benzene at this temperature is 7461 mmHg and its density is 087865 gcm3.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title will ethanol dissolve in carbon tetrachloride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.