Zinc chloride ammonia equation
Zinc Chloride Ammonia Equation. It would be nearly impossible to break those ionicelectrovalent bonds. Convert the chemical names into chemical formulas. Refer to Thermodynamic Properties of Ammonia as an Ideal Gas NSDS-NBS 19. Salt is a very strong bond when it is sitting on your table.
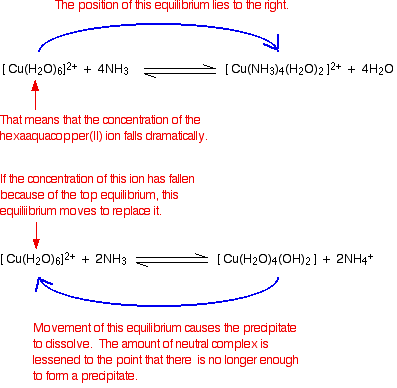 Reactions Of Aqua Ions With Ammonia Solution From chemguide.co.uk
Reactions Of Aqua Ions With Ammonia Solution From chemguide.co.uk
Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100. You want to measure how much water is produced when 120 g of glucose C_6H_12O_6 is burned with enough oxygen. In order to obtain a hydrated form of the compound hydrochloric acid can be used to treat zinc instead of hydrogen chloride. Refer to Thermodynamic Properties of Ammonia as an Ideal Gas NSDS-NBS 19. Zinc Silver nitrate - Zinc nitrate Silver. The reaction between metallic zinc and hydrogen chloride gas yields the anhydrous form of zinc chloride.
Among the elements it has the highest electron.
Look at sodium chloride NaCl one more time. The above checks show that the ideal gas equation can be used with reasonable accuracy to approximate pressure specific volume or temperature if two of the three properties are known. However if you put that salt into some water H 2 O the bonds break very quickly. In order to obtain a hydrated form of the compound hydrochloric acid can be used to treat zinc instead of hydrogen chloride. Refer to Thermodynamic Properties of Ammonia as an Ideal Gas NSDS-NBS 19. Write a balanced chemical equation including the state symbols.
 Source: slideplayer.com
Source: slideplayer.com
Chlorine is a chemical element with the symbol Cl and atomic number 17. It happens easily because of the electrical attraction of the water. The second-lightest of the halogens it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. However if you put that salt into some water H 2 O the bonds break very quickly.
 Source: youtube.com
Source: youtube.com
Chlorine is a yellow-green gas at room temperature. It happens easily because of the electrical attraction of the water. Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100. It would be nearly impossible to break those ionicelectrovalent bonds. Now you have sodium Na and chlorine Cl- ions floating.
 Source: youtube.com
Source: youtube.com
In the superheat area results are typically within 5 of actual. N 2 3H 2 2NH 3. Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia NH 3. Now you have sodium Na and chlorine Cl- ions floating. It would be nearly impossible to break those ionicelectrovalent bonds.
 Source: youtube.com
Source: youtube.com
Barium chloride Potassium sulphate - Barium sulphate Potassium chloride. The chemical equation for this reaction is given by. Hydrochloric acid also reacts with zinc sulphide to form zinc chloride and hydrogen. Among the elements it has the highest electron. Barium chloride Potassium sulphate - Barium sulphate Potassium chloride.
 Source: chemguide.co.uk
Source: chemguide.co.uk
The chemical equation for this reaction is given by. Identify reactants and products and place them in a word equation. Many chemical equations also include phase labels for the substances. Yield actual yieldtheoretical yield 100 So lets say you want to do an experiment in the lab. It happens easily because of the electrical attraction of the water.
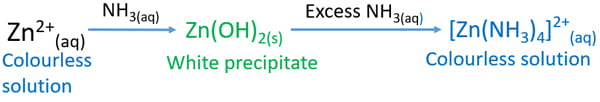 Source: chemistryscl.com
Source: chemistryscl.com
N 2 3H 2 2NH 3. Barium chloride Potassium sulphate - Barium sulphate Potassium chloride. Chlorine is a chemical element with the symbol Cl and atomic number 17. Errors are greatest near the saturation line and at higher pressures. The above checks show that the ideal gas equation can be used with reasonable accuracy to approximate pressure specific volume or temperature if two of the three properties are known.
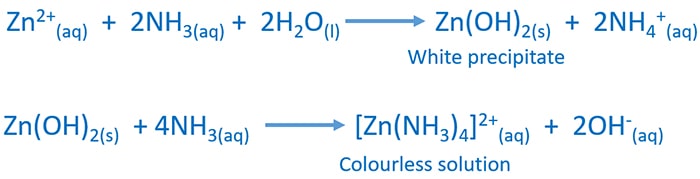 Source: chemistryscl.com
Source: chemistryscl.com
The reaction between metallic zinc and hydrogen chloride gas yields the anhydrous form of zinc chloride. Among the elements it has the highest electron. Look at sodium chloride NaCl one more time. Refer to Thermodynamic Properties of Ammonia as an Ideal Gas NSDS-NBS 19. Calcium hydroxide Carbon dioxide - Calcium carbonate Water.
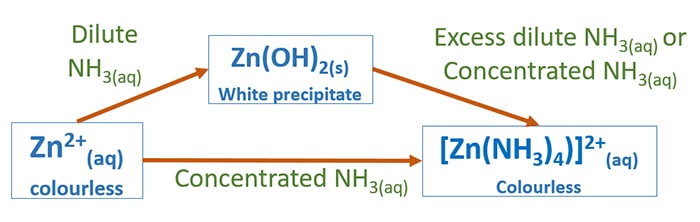 Source: chemistryscl.com
Source: chemistryscl.com
Write the balanced chemical equation for the following reactions. You want to measure how much water is produced when 120 g of glucose C_6H_12O_6 is burned with enough oxygen. Calcium hydroxide Carbon dioxide - Calcium carbonate Water. Now you have sodium Na and chlorine Cl- ions floating. It would be nearly impossible to break those ionicelectrovalent bonds.
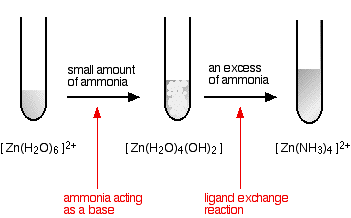 Source: chemguide.co.uk
Source: chemguide.co.uk
Calcium hydroxide Carbon dioxide - Calcium carbonate Water. Chlorine is a yellow-green gas at room temperature. Write a balanced chemical equation including the state symbols. Special conditions such as temperature may also be listed above the arrow. Aluminium Copper chloride - Aluminium chloride Copper.
 Source: brainly.in
Source: brainly.in
Yield actual yieldtheoretical yield 100 So lets say you want to do an experiment in the lab. The second-lightest of the halogens it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Write the balanced chemical equation for the following reactions. Errors are greatest near the saturation line and at higher pressures. In order to obtain a hydrated form of the compound hydrochloric acid can be used to treat zinc instead of hydrogen chloride.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title zinc chloride ammonia equation by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






