Zinc nitrate sodium nitrate
Zinc Nitrate Sodium Nitrate. Sodium nitrate Sodium nitrate forms through the evaporation of water in desert areas. As a consequence the low cost precursors such as zinc nitrate and sodium hydroxide to synthesize the ZnO nanoparticles ca. 40 nm were used. Nitrogen and sulfur are essential to the supply of amino acids and proteins.
 When Zinc Nitrate Reacts With Sodium Sulphide Then A Soluble Salts Zinc Sulphide And Sodium Nitrate Are Formed B Soluble Salt Zinc Sulphide And Insoluble Salt Sodium Nitrate Is Formed C From toppr.com
When Zinc Nitrate Reacts With Sodium Sulphide Then A Soluble Salts Zinc Sulphide And Sodium Nitrate Are Formed B Soluble Salt Zinc Sulphide And Insoluble Salt Sodium Nitrate Is Formed C From toppr.com
A finely divided mixture of potassium ferrocyanide and cupric nitrate exploded when dried at 220 C Chem. Contrairement à dautres sels de plombII il est soluble dans leauSon usage principal depuis le Moyen Âge sous le nom de plumb dulcis a été comme matière première de nombreux pigmentsDepuis le XX e siècle il est utilisé comme. Le nitrate de plombII est un sel inorganique de plomb et dacide nitriqueCest un cristal incolore ou une poudre blanche et un oxydant stable et fort. Mixtures of CUPRIC NITRATE with alkyl esters may explode owing to the formation of alkyl nitrates. Sodium nitrate Sodium nitrate forms through the evaporation of water in desert areas. For example some valuable metallic minerals such as gold silver iron zinc and uranium are found in Western Desert in Australia.
Le nitrate de plombII est un sel inorganique de plomb et dacide nitriqueCest un cristal incolore ou une poudre blanche et un oxydant stable et fort.
This is due to special geological processes and climate factors in the desert can preserve and enhance mineral deposits. Nitrogen and sulfur are essential to the supply of amino acids and proteins. Sodium reacts rapidly with water snow and ice to produce sodium hydroxide. As a consequence the low cost precursors such as zinc nitrate and sodium hydroxide to synthesize the ZnO nanoparticles ca. Phosphorus is used in photosynthesis and overall. Sodium nitrate Sodium nitrate forms through the evaporation of water in desert areas.
 Source: scielo.br
Source: scielo.br
Mixtures with phosphorus tinII chloride or other reducing agents may react explosively Bretherick 1979 p. In order to reduce the agglomeration among the smaller particles the starch molecule which contains many O-H functional groups and could bind surface of nanoparticles in initial nucleation stage was used. Mixtures with phosphorus tinII chloride or other reducing agents may react explosively Bretherick 1979 p. Sodium nitrate Sodium nitrate forms through the evaporation of water in desert areas. For example some valuable metallic minerals such as gold silver iron zinc and uranium are found in Western Desert in Australia.
 Source: youtube.com
Source: youtube.com
The preparation of BaNO32 by double conversion of barium chloride and sodium nitrate or calcium nitrate is described in the Eastern European patent literature. Two equivalent images of the. For example some valuable metallic minerals such as gold silver iron zinc and uranium are found in Western Desert in Australia. Another process of controlled precipitation of. This is due to special geological processes and climate factors in the desert can preserve and enhance mineral deposits.
 Source: toppr.com
Source: toppr.com
Each element involved in these nutrients provides a different benefit. Contrairement à dautres sels de plombII il est soluble dans leauSon usage principal depuis le Moyen Âge sous le nom de plumb dulcis a été comme matière première de nombreux pigmentsDepuis le XX e siècle il est utilisé comme. In order to reduce the agglomeration among the smaller particles the starch molecule which contains many O-H functional groups and could bind surface of nanoparticles in initial nucleation stage was used. This is due to special geological processes and climate factors in the desert can preserve and enhance mineral deposits. Two equivalent images of the.
 Source: bartleby.com
Source: bartleby.com
The richest cache of. The most important sodium compounds are table salt NaCl soda ash Na 2 CO 3 baking soda NaHCO 3 caustic soda NaOH sodium nitrate NaNO 3 di- and tri-sodium phosphates sodium thiosulfate Na 2 S 2 O 3 5H 2 O and borax Na 2 B 4 O 7 10H 2 O. After carefully removing any sulfur formed and the heavy metals the barium nitrate is crystallized at low temperatures. Sodium nitrate Sodium nitrate forms through the evaporation of water in desert areas. As a consequence the low cost precursors such as zinc nitrate and sodium hydroxide to synthesize the ZnO nanoparticles ca.
 Source: fertrell.com
Source: fertrell.com
Mixtures with phosphorus tinII chloride or other reducing agents may react explosively Bretherick 1979 p. Mixtures of CUPRIC NITRATE with alkyl esters may explode owing to the formation of alkyl nitrates. The essential nutrients used include calcium nitrate potassium sulphate potassium nitrate mono potassium phosphate and magnesium sulphate. The most important sodium compounds are table salt NaCl soda ash Na 2 CO 3 baking soda NaHCO 3 caustic soda NaOH sodium nitrate NaNO 3 di- and tri-sodium phosphates sodium thiosulfate Na 2 S 2 O 3 5H 2 O and borax Na 2 B 4 O 7 10H 2 O. Another process of controlled precipitation of.
 Source: youtube.com
Source: youtube.com
A finely divided mixture of potassium ferrocyanide and cupric nitrate exploded when dried at 220 C Chem. Sodium does not react with nitrogen even at very high temperatures but reacts with ammonia to form sodium amide. Le nitrate de plombII est un sel inorganique de plomb et dacide nitriqueCest un cristal incolore ou une poudre blanche et un oxydant stable et fort. When metallic sodium is exposed to air it loses its silver appearance and develops an opaque grey colour layer which is a coating of sodium oxide. A finely divided mixture of potassium ferrocyanide and cupric nitrate exploded when dried at 220 C Chem.
 Source: youtube.com
Source: youtube.com
Phosphorus is used in photosynthesis and overall. Each element involved in these nutrients provides a different benefit. The essential nutrients used include calcium nitrate potassium sulphate potassium nitrate mono potassium phosphate and magnesium sulphate. Le nitrate de plombII est un sel inorganique de plomb et dacide nitriqueCest un cristal incolore ou une poudre blanche et un oxydant stable et fort. Another process of controlled precipitation of.
 Source: researchgate.net
Source: researchgate.net
Le nitrate de plombII est un sel inorganique de plomb et dacide nitriqueCest un cristal incolore ou une poudre blanche et un oxydant stable et fort. After carefully removing any sulfur formed and the heavy metals the barium nitrate is crystallized at low temperatures. Two equivalent images of the. In order to reduce the agglomeration among the smaller particles the starch molecule which contains many O-H functional groups and could bind surface of nanoparticles in initial nucleation stage was used. Sodium nitrate Sodium nitrate forms through the evaporation of water in desert areas.
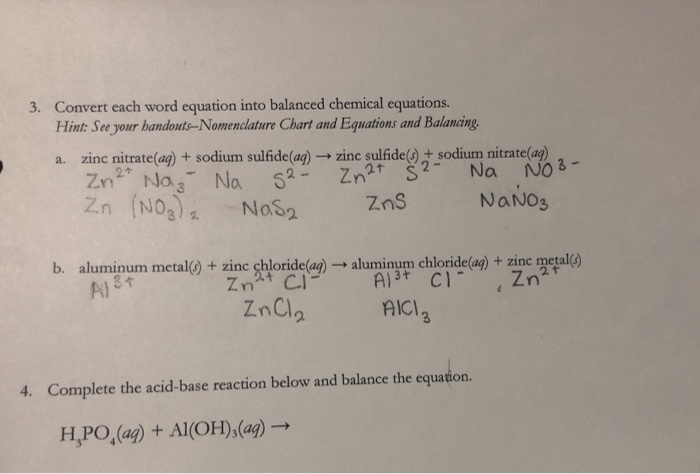 Source: chegg.com
Source: chegg.com
Sodium does not react with nitrogen even at very high temperatures but reacts with ammonia to form sodium amide. Sodium nitrate Sodium nitrate forms through the evaporation of water in desert areas. The essential nutrients used include calcium nitrate potassium sulphate potassium nitrate mono potassium phosphate and magnesium sulphate. A finely divided mixture of potassium ferrocyanide and cupric nitrate exploded when dried at 220 C Chem. This is due to special geological processes and climate factors in the desert can preserve and enhance mineral deposits.
 Source: toppr.com
Source: toppr.com
Contrairement à dautres sels de plombII il est soluble dans leauSon usage principal depuis le Moyen Âge sous le nom de plumb dulcis a été comme matière première de nombreux pigmentsDepuis le XX e siècle il est utilisé comme. Sodium reacts rapidly with water snow and ice to produce sodium hydroxide. For example some valuable metallic minerals such as gold silver iron zinc and uranium are found in Western Desert in Australia. As a consequence the low cost precursors such as zinc nitrate and sodium hydroxide to synthesize the ZnO nanoparticles ca. Each element involved in these nutrients provides a different benefit.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title zinc nitrate sodium nitrate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.