Zinc reacting with copper chloride
Zinc Reacting With Copper Chloride. Bronze is also used frequently in ship-building because it is resistant to corrosion from sea water. Titanium is much lighter and less dense than steel but as strong. Active carbon uses the physical adsorption process whereby Vanderwaals attractive forces pull the solute contamination out of the solution and onto its surface. Additionally when zinc chloride is used as the Lewis acid instead of aluminum chloride for example or when the carbon monoxide is not used at high pressure the presence of traces of copperI chloride or nickelII chloride co-catalyst is often necessary.
 Single Displacement Reaction Zinc And Copper Ii Ion Redox Chemdemos From chemdemos.uoregon.edu
Single Displacement Reaction Zinc And Copper Ii Ion Redox Chemdemos From chemdemos.uoregon.edu
Active carbon uses the physical adsorption process whereby Vanderwaals attractive forces pull the solute contamination out of the solution and onto its surface. Titanium is much lighter and less dense than steel but as strong. The transition metal co-catalyst may server as a carrier by first forming reacting with CO to form a carbonyl complex which is then. Ammonium chloride is an inorganic compound with the formula NH 4 Cl and a white crystalline salt that is highly soluble in water. It is also found around some types of volcanic vents. Bronze is also used frequently in ship-building because it is resistant to corrosion from sea water.
Its also very resistant to corrosion.
It is also found around some types of volcanic vents. Its also very resistant to corrosion. Titanium is much lighter and less dense than steel but as strong. Copper MCL 13 mgL EPA US 2006. The efficiency of the adsorption depends on. Additionally when zinc chloride is used as the Lewis acid instead of aluminum chloride for example or when the carbon monoxide is not used at high pressure the presence of traces of copperI chloride or nickelII chloride co-catalyst is often necessary.
 Source: spark.adobe.com
Source: spark.adobe.com
The efficiency of the adsorption depends on. The transition metal co-catalyst may server as a carrier by first forming reacting with CO to form a carbonyl complex which is then. Other alloys like brass copper and zinc and bronze copper and tin are easy to shape and beautiful to look at. Footprints-Science - 1000 GCSE science animations quizzes drag and drops anagrams wordsearches and more. Active carbon uses the physical adsorption process whereby Vanderwaals attractive forces pull the solute contamination out of the solution and onto its surface.
 Source: chemdemos.uoregon.edu
Source: chemdemos.uoregon.edu
Ammonium chloride is an inorganic compound with the formula NH 4 Cl and a white crystalline salt that is highly soluble in water. Other alloys like brass copper and zinc and bronze copper and tin are easy to shape and beautiful to look at. And although heavier than aluminum its also twice as strong. It is also found around some types of volcanic vents. Why does the reaction of iron and copper chloride occur.
 Source: youtube.com
Source: youtube.com
The efficiency of the adsorption depends on. Butylated reaction product of p-cresol and dicyclopentadiene produced by reacting p-cresol and dicyclopentadiene in an approximate mole ratio of 15 to 1 respectively followed by alkylation with isobutylene so that the butyl content of the final product is not less than 18 percent. Why does the reaction of iron and copper chloride occur Why does the reaction of iron and copper chloride occur. As components of nonfood articles complying with 175105 and 1772600c4iii of this. Its also very resistant to corrosion.
 Source: chemdemos.uoregon.edu
Source: chemdemos.uoregon.edu
The efficiency of the adsorption depends on. The transition metal co-catalyst may server as a carrier by first forming reacting with CO to form a carbonyl complex which is then. Solutions of ammonium chloride are mildly acidic. Zinc chloride and the mixture carbonized at an elevated temperature followed by the removal of activating agent by water washing chemical method. Copper MCL 13 mgL EPA US 2006.
 Source: spark.adobe.com
Source: spark.adobe.com
In its naturally occurring mineralogic form it is known as sal ammoniacThe mineral is commonly formed on burning coal dumps from condensation of coal-derived gases. Why does the reaction of iron and copper chloride occur Why does the reaction of iron and copper chloride occur. As components of nonfood articles complying with 175105 and 1772600c4iii of this. In its naturally occurring mineralogic form it is known as sal ammoniacThe mineral is commonly formed on burning coal dumps from condensation of coal-derived gases. The transition metal co-catalyst may server as a carrier by first forming reacting with CO to form a carbonyl complex which is then.
 Source: youtube.com
Source: youtube.com
Ammonium chloride is an inorganic compound with the formula NH 4 Cl and a white crystalline salt that is highly soluble in water. As components of nonfood articles complying with 175105 and 1772600c4iii of this. Zinc chloride and the mixture carbonized at an elevated temperature followed by the removal of activating agent by water washing chemical method. Why does the reaction of iron and copper chloride occur Why does the reaction of iron and copper chloride occur. Footprints-Science - 1000 GCSE science animations quizzes drag and drops anagrams wordsearches and more.
 Source: youtube.com
Source: youtube.com
The transition metal co-catalyst may server as a carrier by first forming reacting with CO to form a carbonyl complex which is then. Solutions of ammonium chloride are mildly acidic. Its also very resistant to corrosion. Zinc chloride and the mixture carbonized at an elevated temperature followed by the removal of activating agent by water washing chemical method. The efficiency of the adsorption depends on.
 Source: spark.adobe.com
Source: spark.adobe.com
It is also found around some types of volcanic vents. Its also very resistant to corrosion. Why does the reaction of iron and copper chloride occur. It is also found around some types of volcanic vents. Titanium is much lighter and less dense than steel but as strong.
 Source: chemdemos.uoregon.edu
Source: chemdemos.uoregon.edu
Additionally when zinc chloride is used as the Lewis acid instead of aluminum chloride for example or when the carbon monoxide is not used at high pressure the presence of traces of copperI chloride or nickelII chloride co-catalyst is often necessary. Footprints-Science - 1000 GCSE science animations quizzes drag and drops anagrams wordsearches and more. Copper MCL 13 mgL EPA US 2006. Ammonium chloride is an inorganic compound with the formula NH 4 Cl and a white crystalline salt that is highly soluble in water. Additionally when zinc chloride is used as the Lewis acid instead of aluminum chloride for example or when the carbon monoxide is not used at high pressure the presence of traces of copperI chloride or nickelII chloride co-catalyst is often necessary.
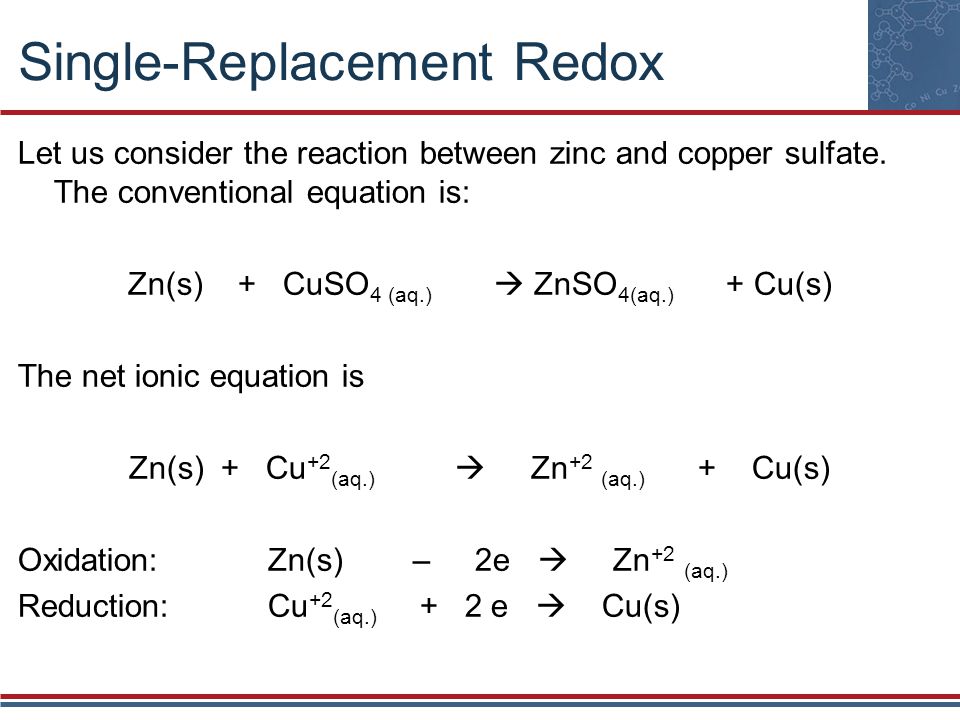 Source: schoolworkhelper.net
Source: schoolworkhelper.net
The efficiency of the adsorption depends on. And although heavier than aluminum its also twice as strong. Active carbon uses the physical adsorption process whereby Vanderwaals attractive forces pull the solute contamination out of the solution and onto its surface. Copper MCL 13 mgL EPA US 2006. Footprints-Science - 1000 GCSE science animations quizzes drag and drops anagrams wordsearches and more.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title zinc reacting with copper chloride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.